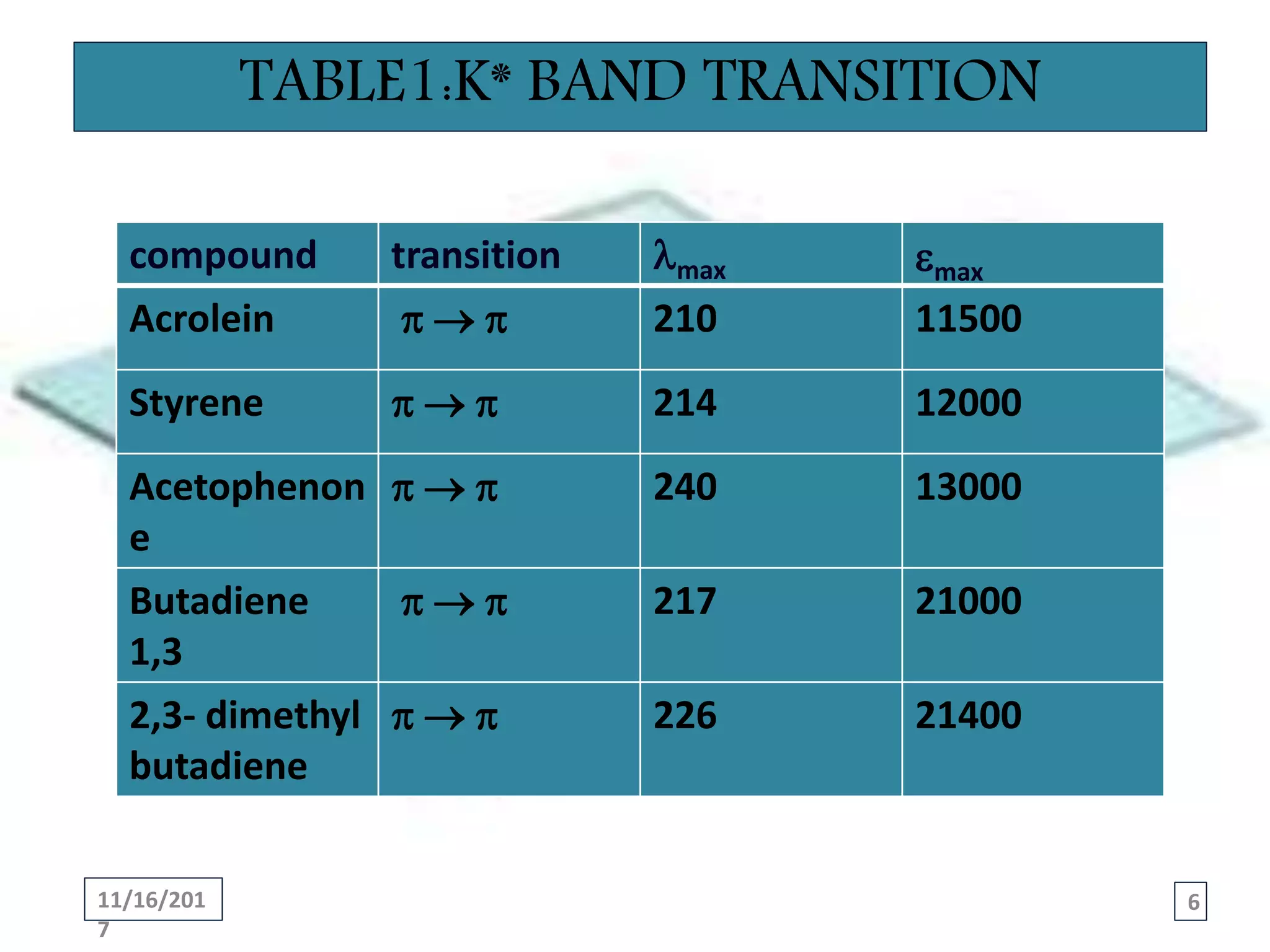
This presentation summarizes the main types of absorption bands found in spectroscopy: K*, R*, B-, and E-bands. Each band corresponds to a different electronic transition. The K* band corresponds to a π → π* transition in conjugated systems. The R* band is a n → π* transition in unsaturated compounds containing heteroatoms. The B-band is a π → π* transition in aromatic compounds. Finally, the E-bands are π → π* transitions characterized by two bands, E1 at lower wavelength and E2 at higher wavelength, found in benzenoid compounds. Examples are provided of characteristic wavelengths and intensities for each type of transition.