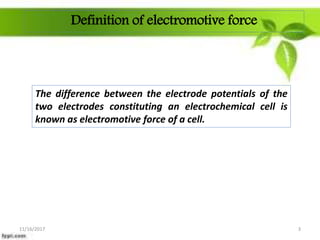
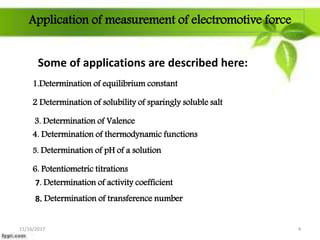
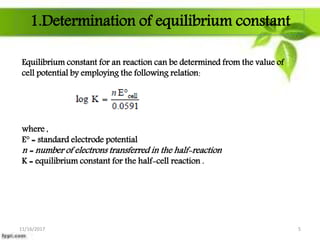
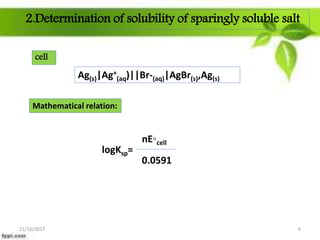
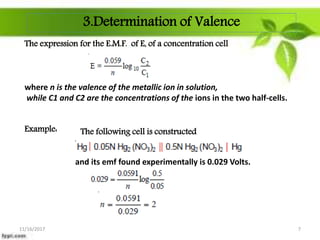
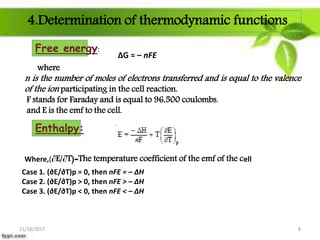
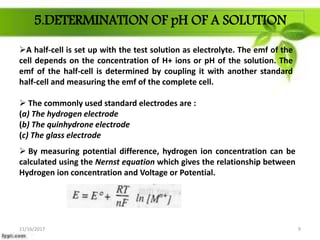
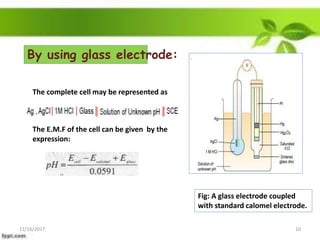
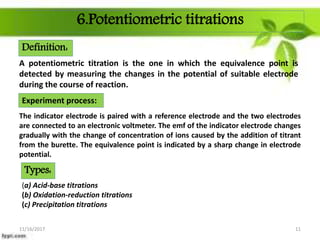
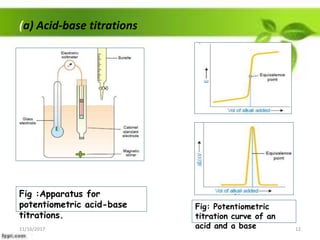
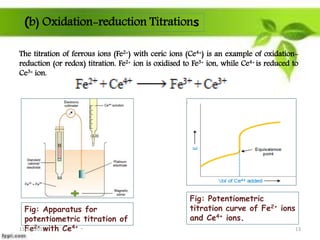
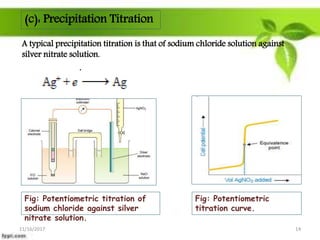
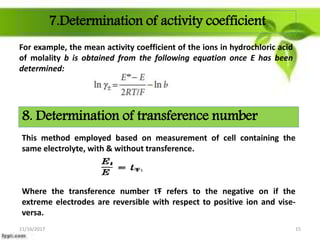
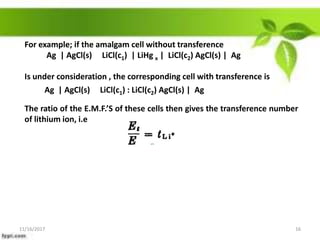
This presentation discusses the application of electromotive force (E.M.F.) measurement. It describes eight applications: 1) determination of equilibrium constants, 2) determination of solubility of sparingly soluble salts, 3) determination of valence, 4) determination of thermodynamic functions, 5) determination of pH of a solution, 6) potentiometric titrations, 7) determination of activity coefficients, and 8) determination of transference numbers. Potentiometric titration techniques are discussed for acid-base, oxidation-reduction, and precipitation titrations. Determination of E.M.F. using cells allows calculation of various chemical properties and equilibrium constants.