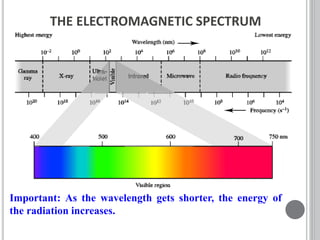
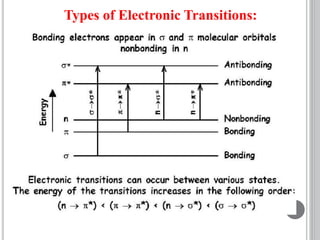
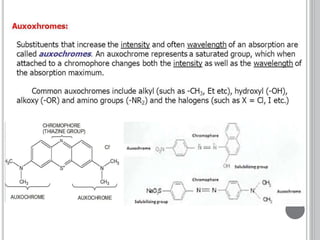
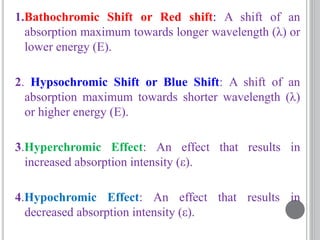
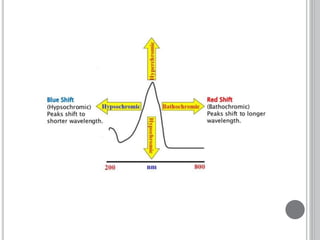
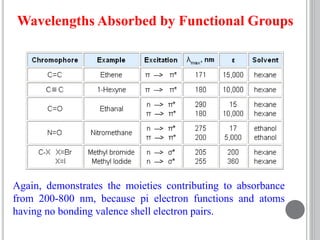
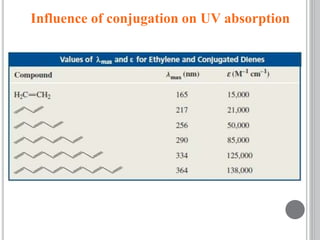
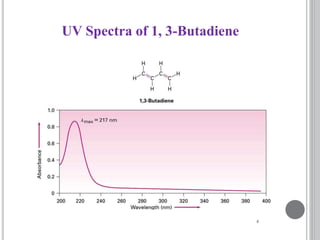
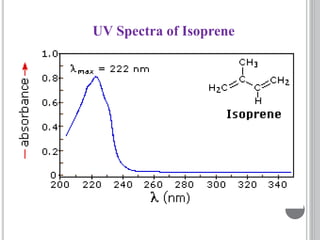
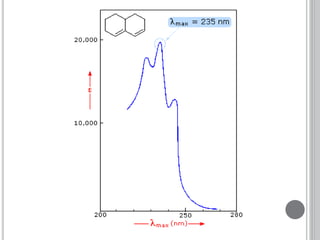
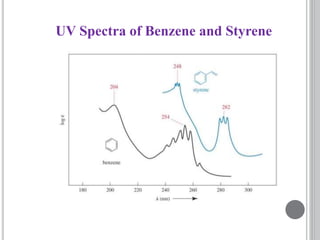
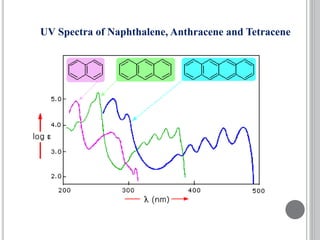
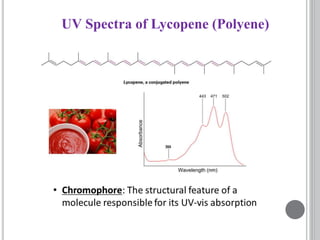
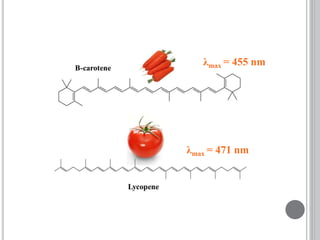
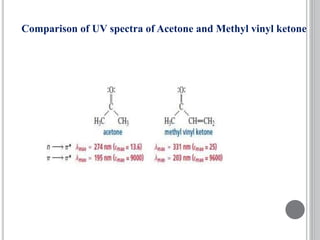
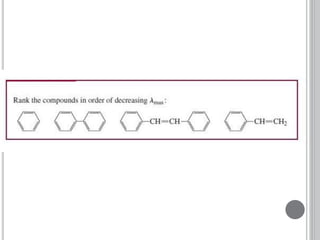
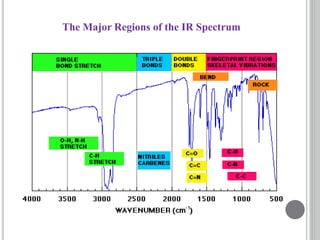
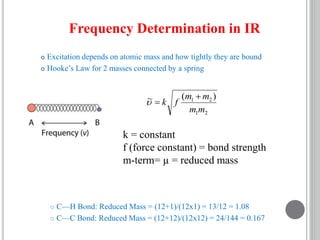
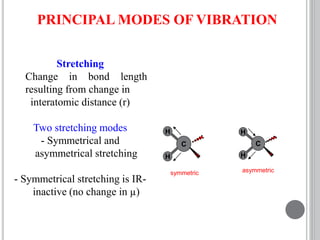
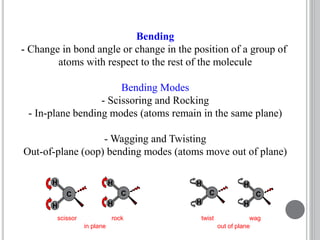
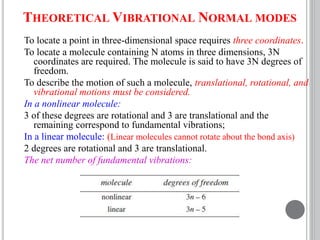
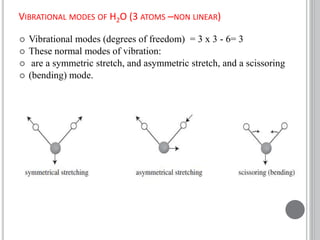
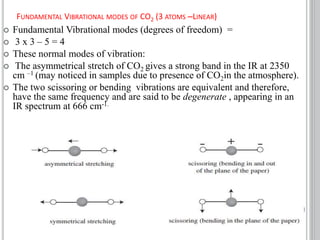
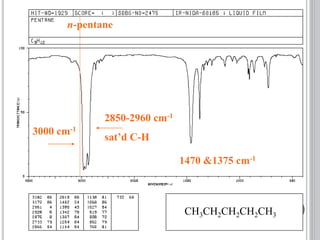
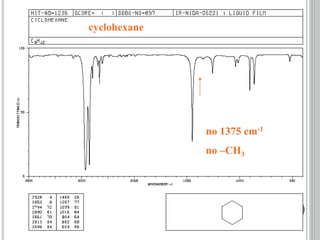
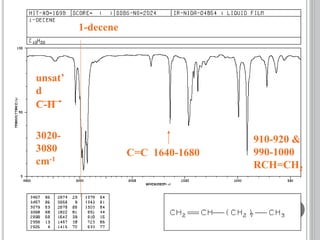
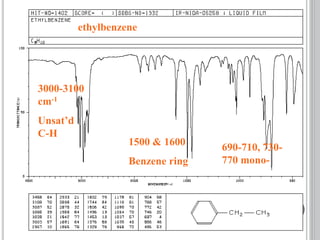
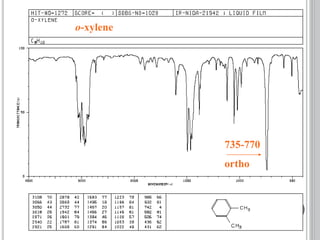
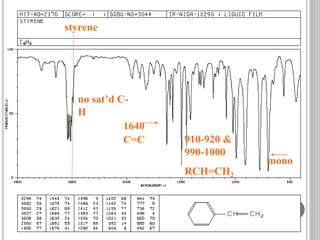
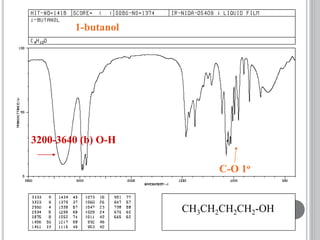
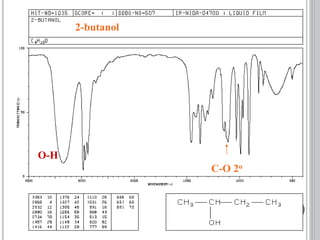
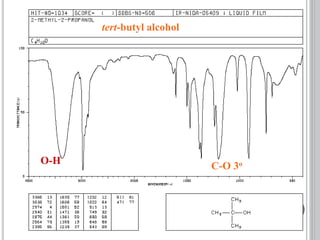
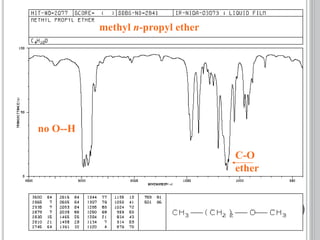
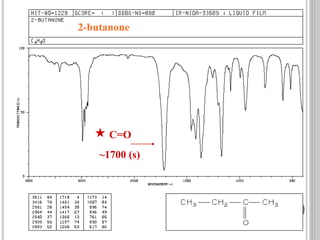
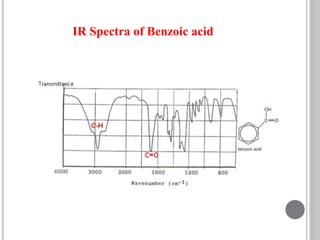
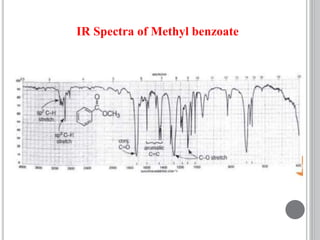
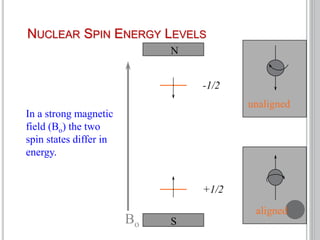
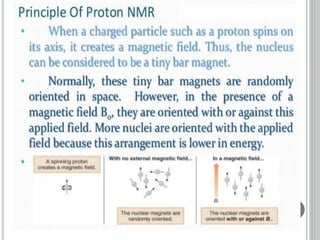
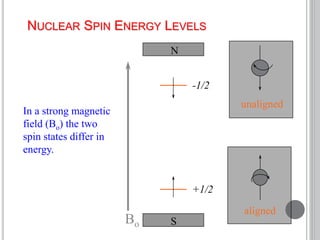
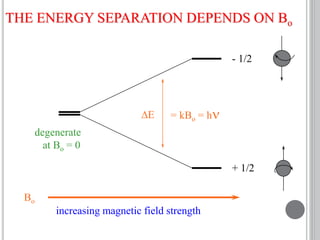
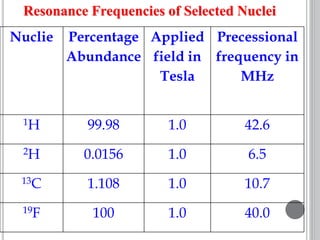
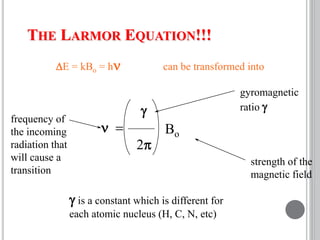
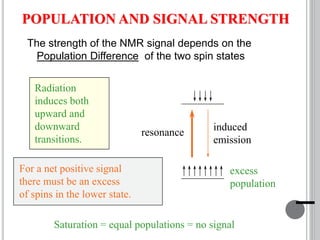
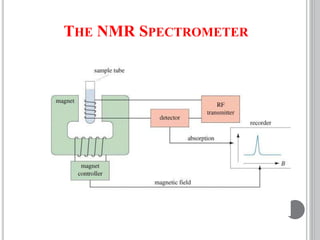
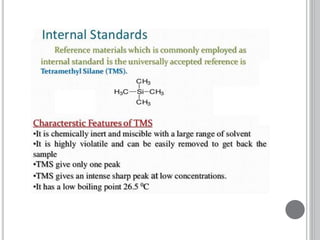
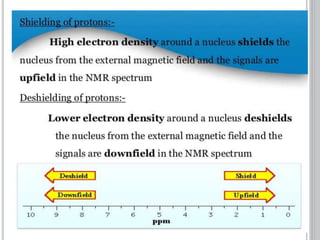
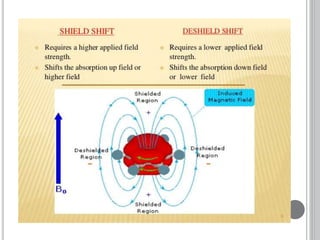
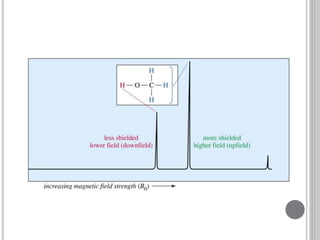
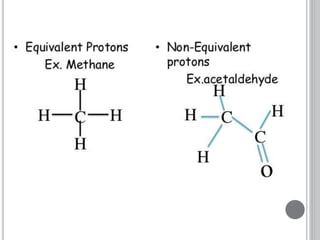
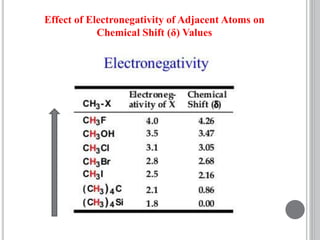
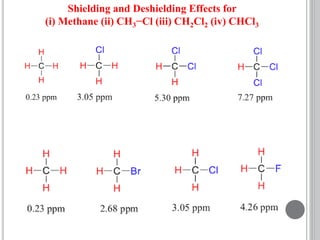
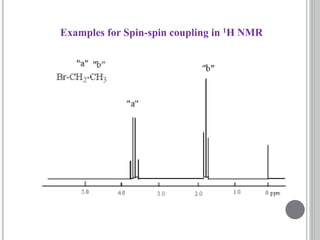
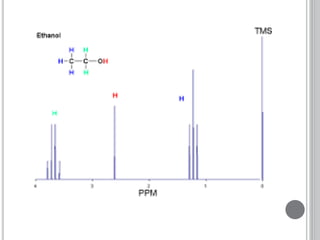
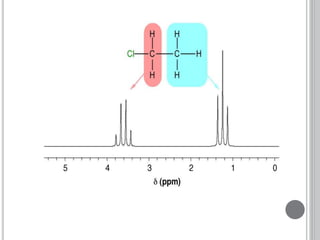
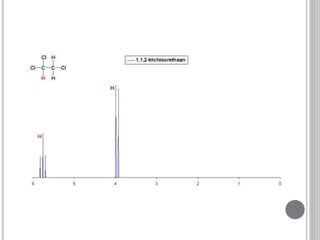
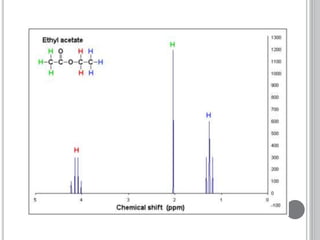
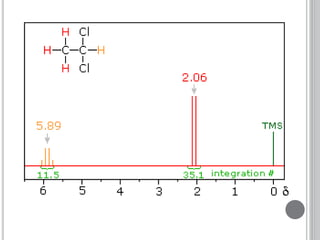
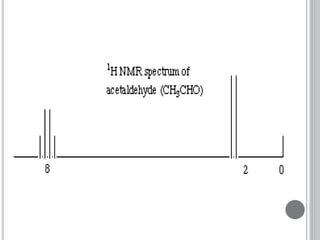
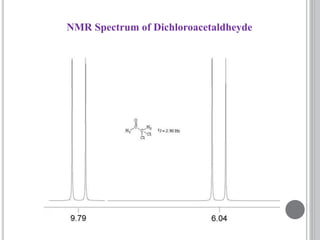
This document provides an overview of various spectroscopy techniques including UV-Vis, IR, and NMR spectroscopy. It discusses key concepts like electromagnetic radiation, photon energy, and the electromagnetic spectrum. It describes the interactions between electromagnetic radiation and matter that are measured in different spectroscopy methods. It also provides examples of spectra for organic compounds and explanations of spectral features.