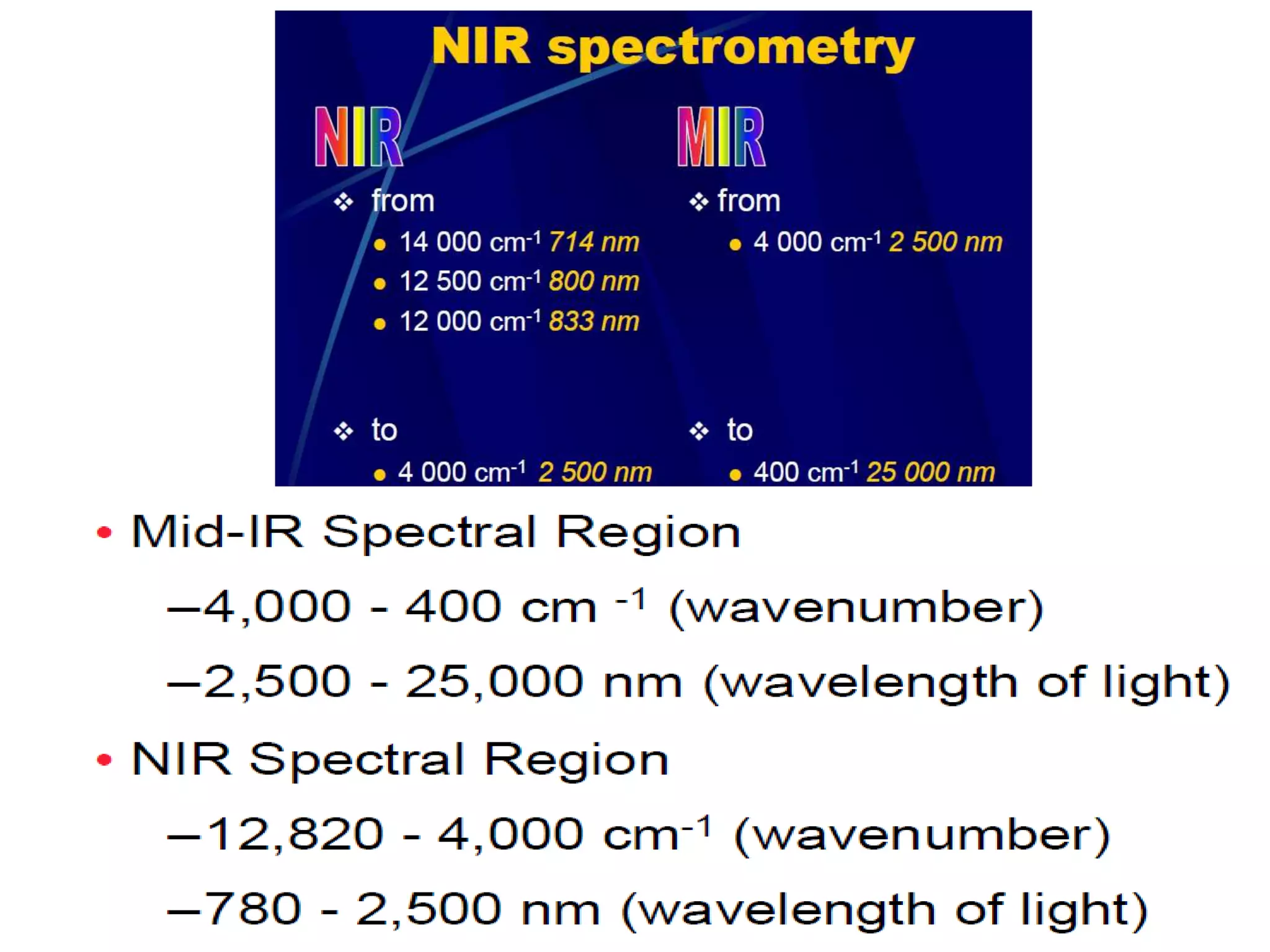
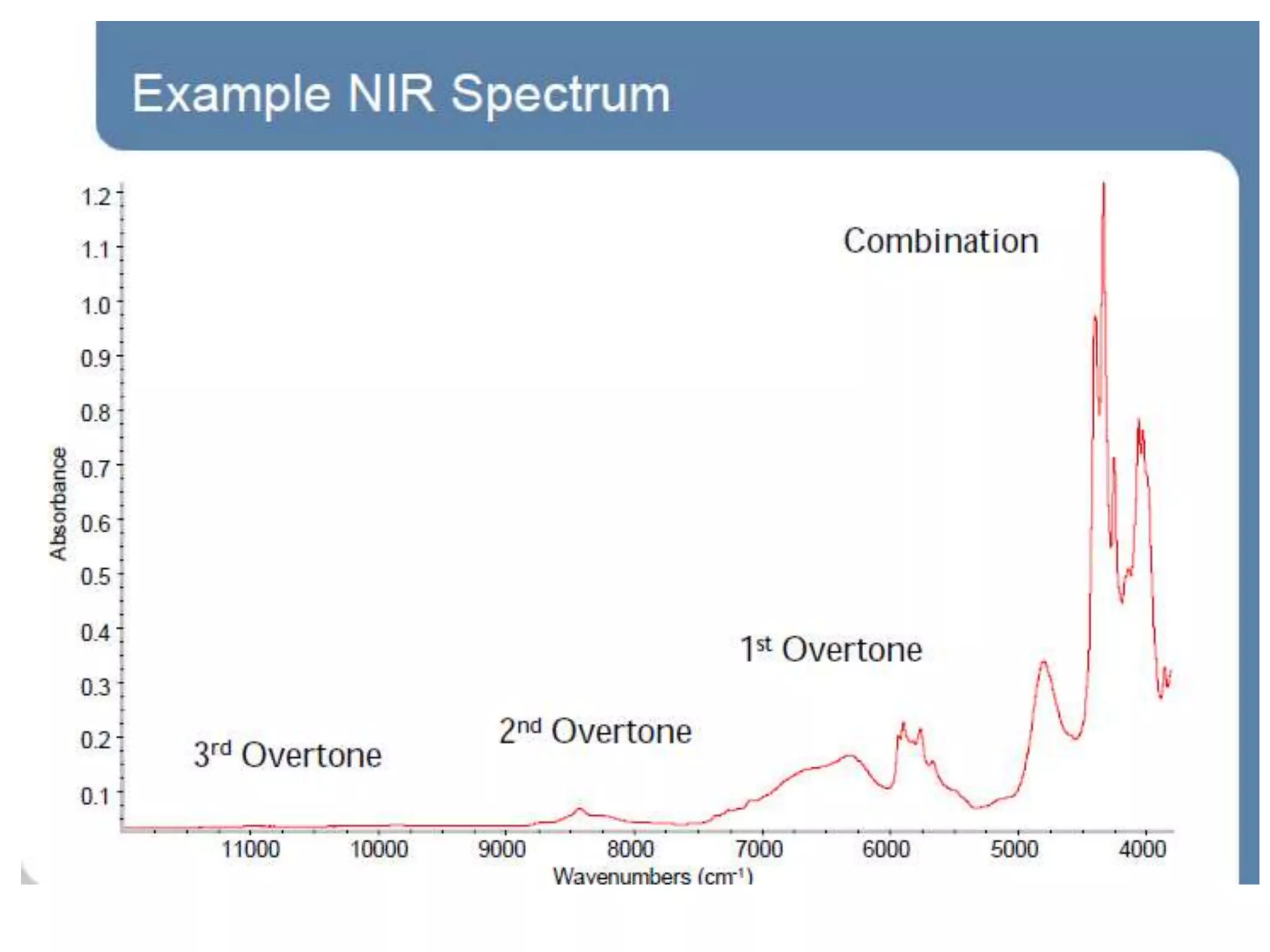
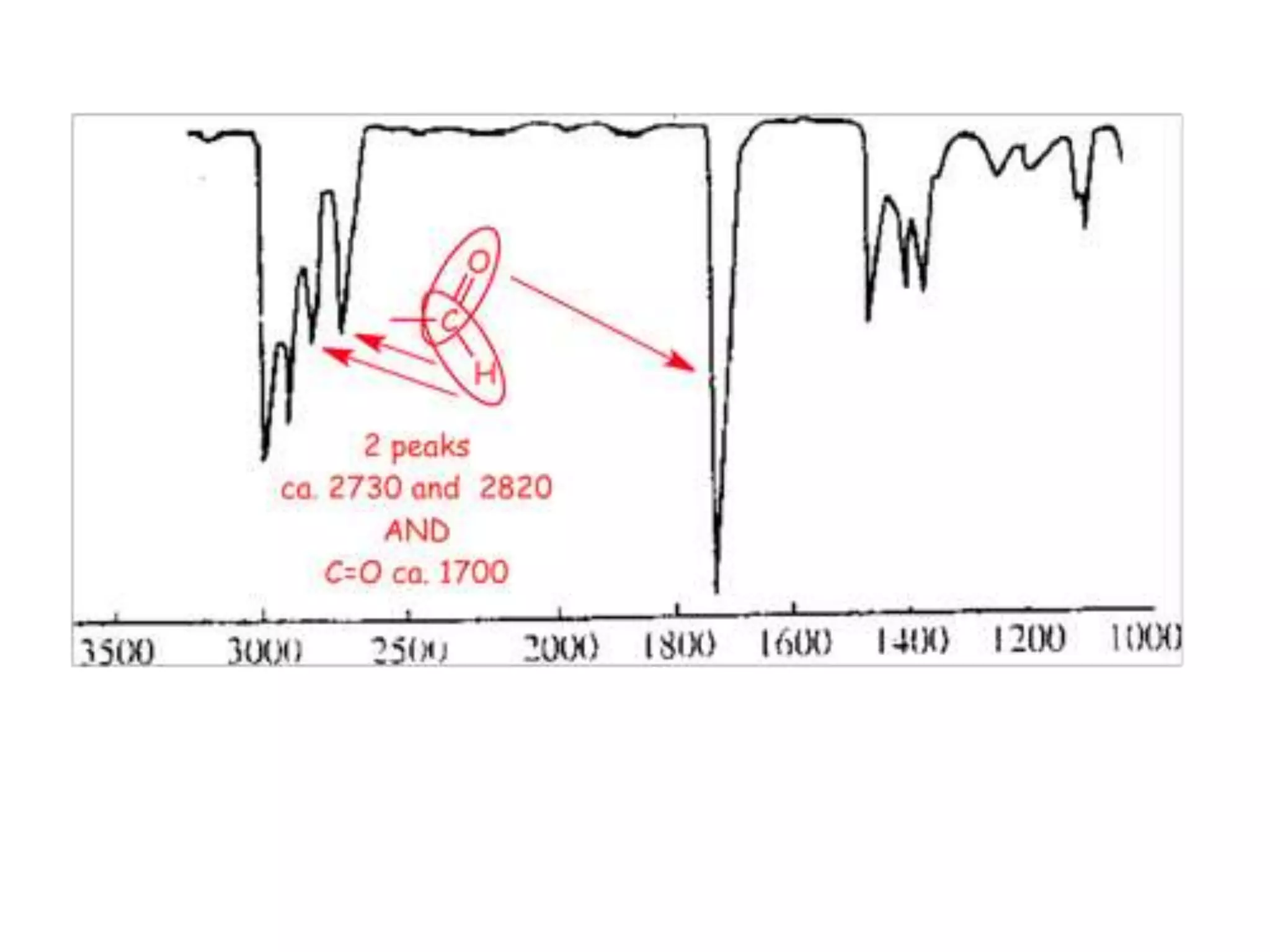
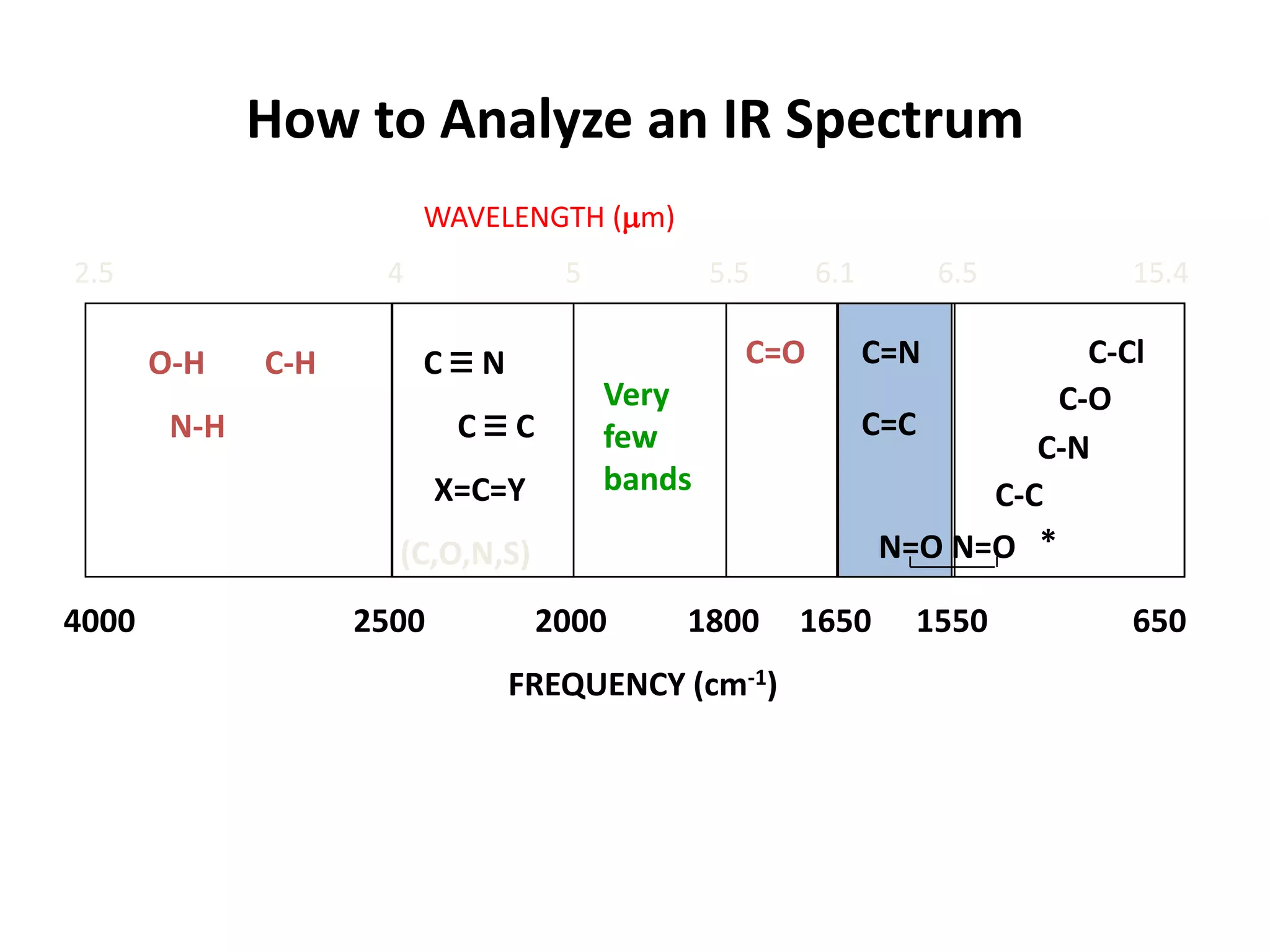
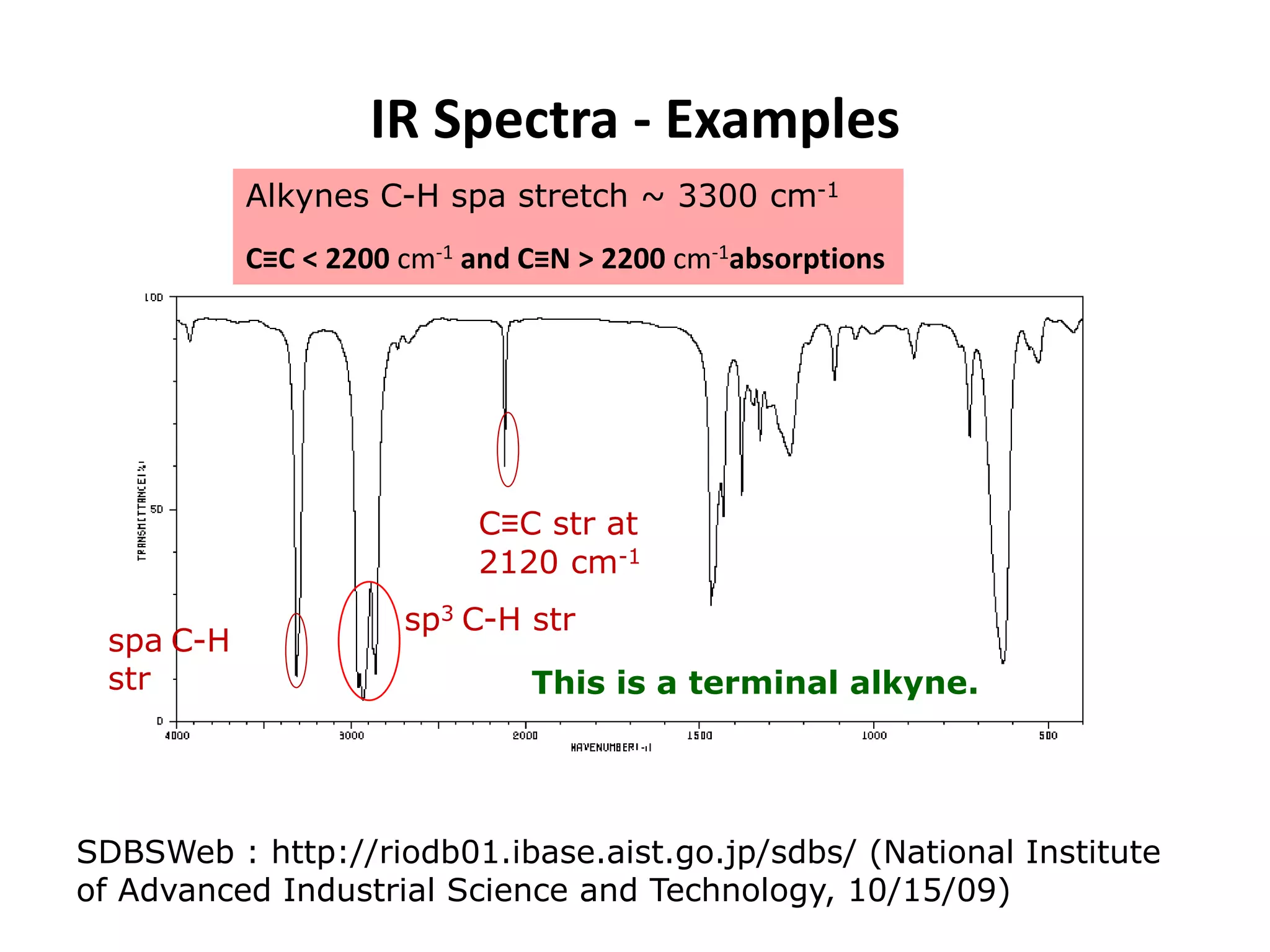
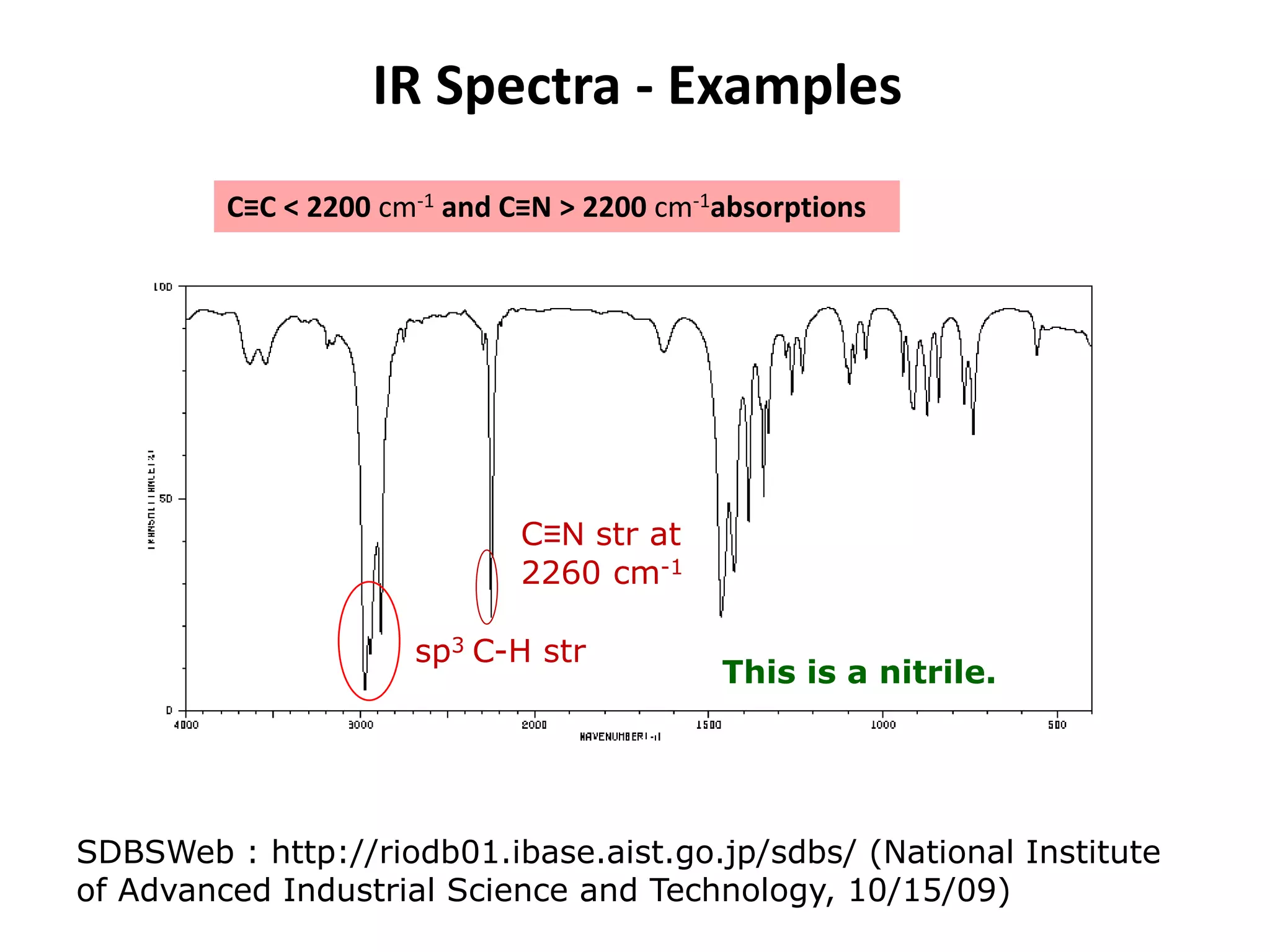
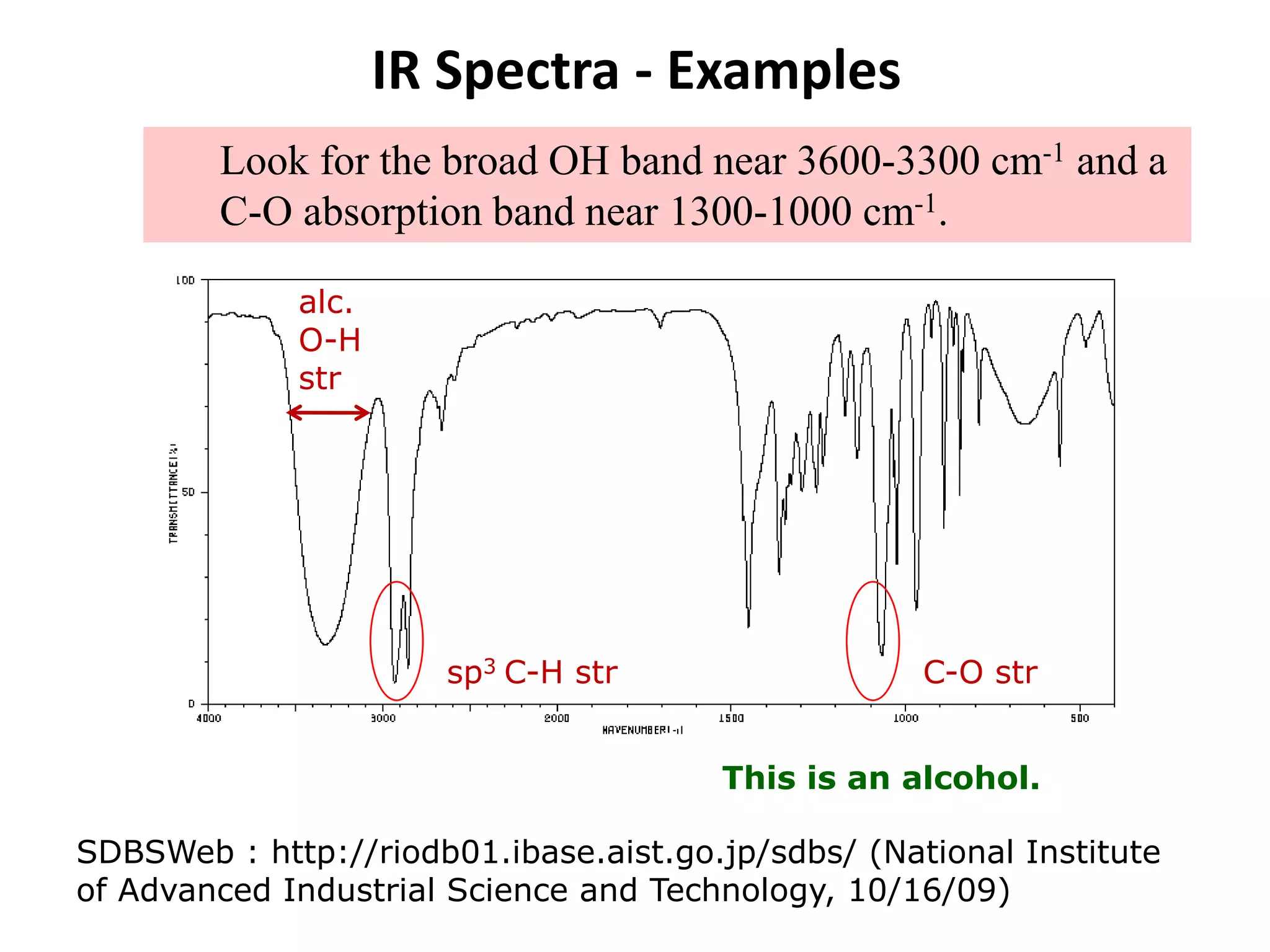
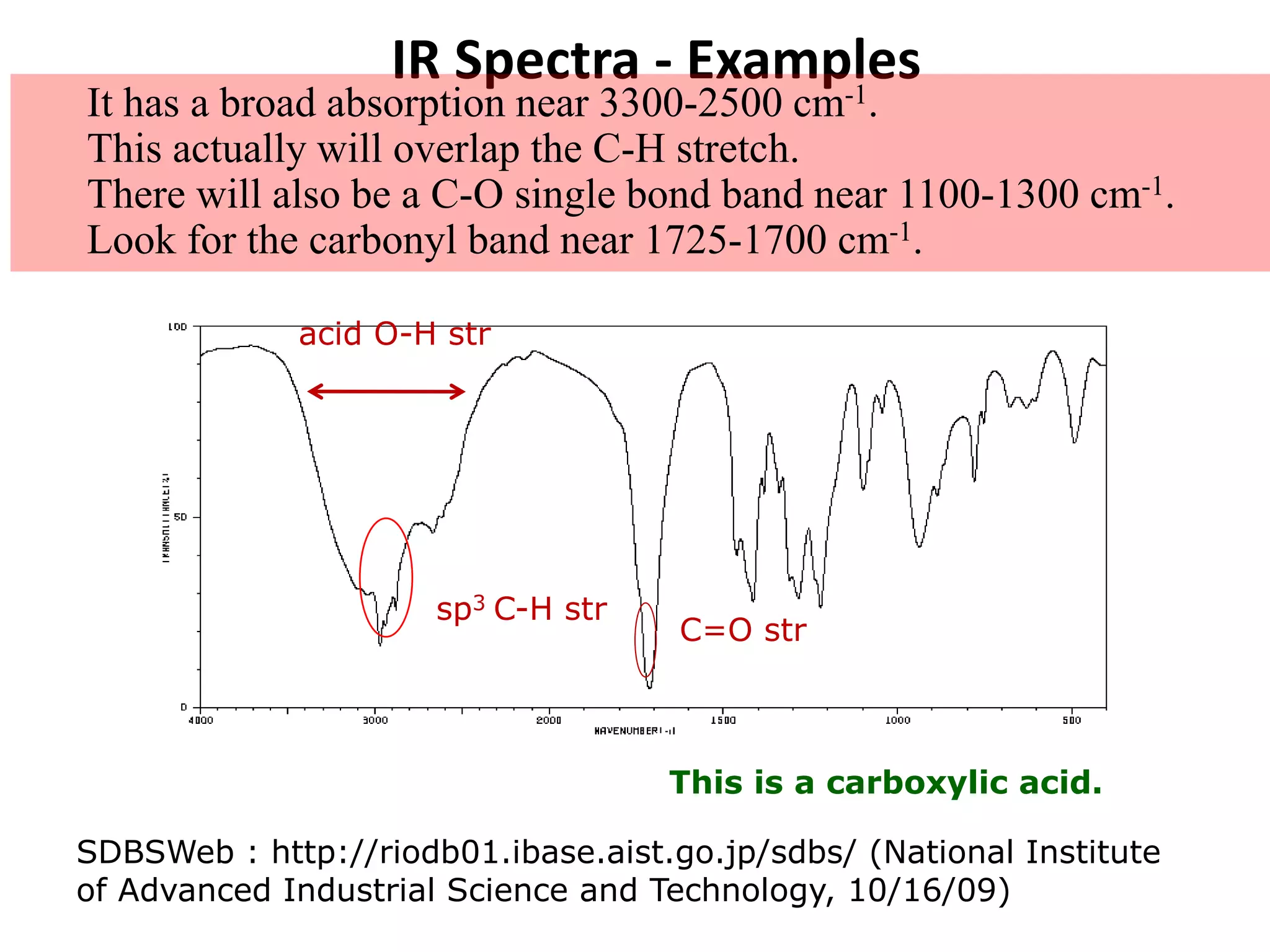
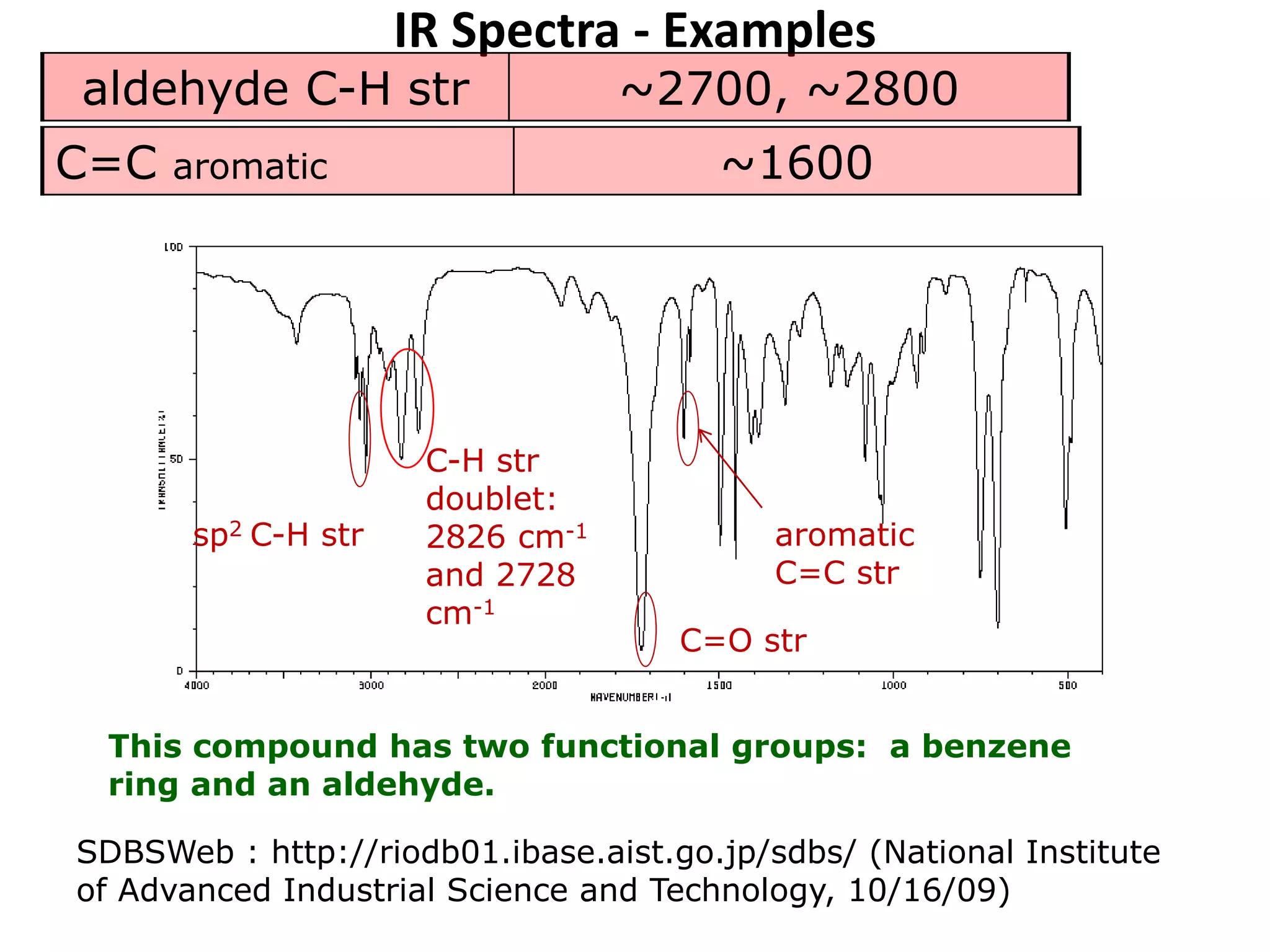
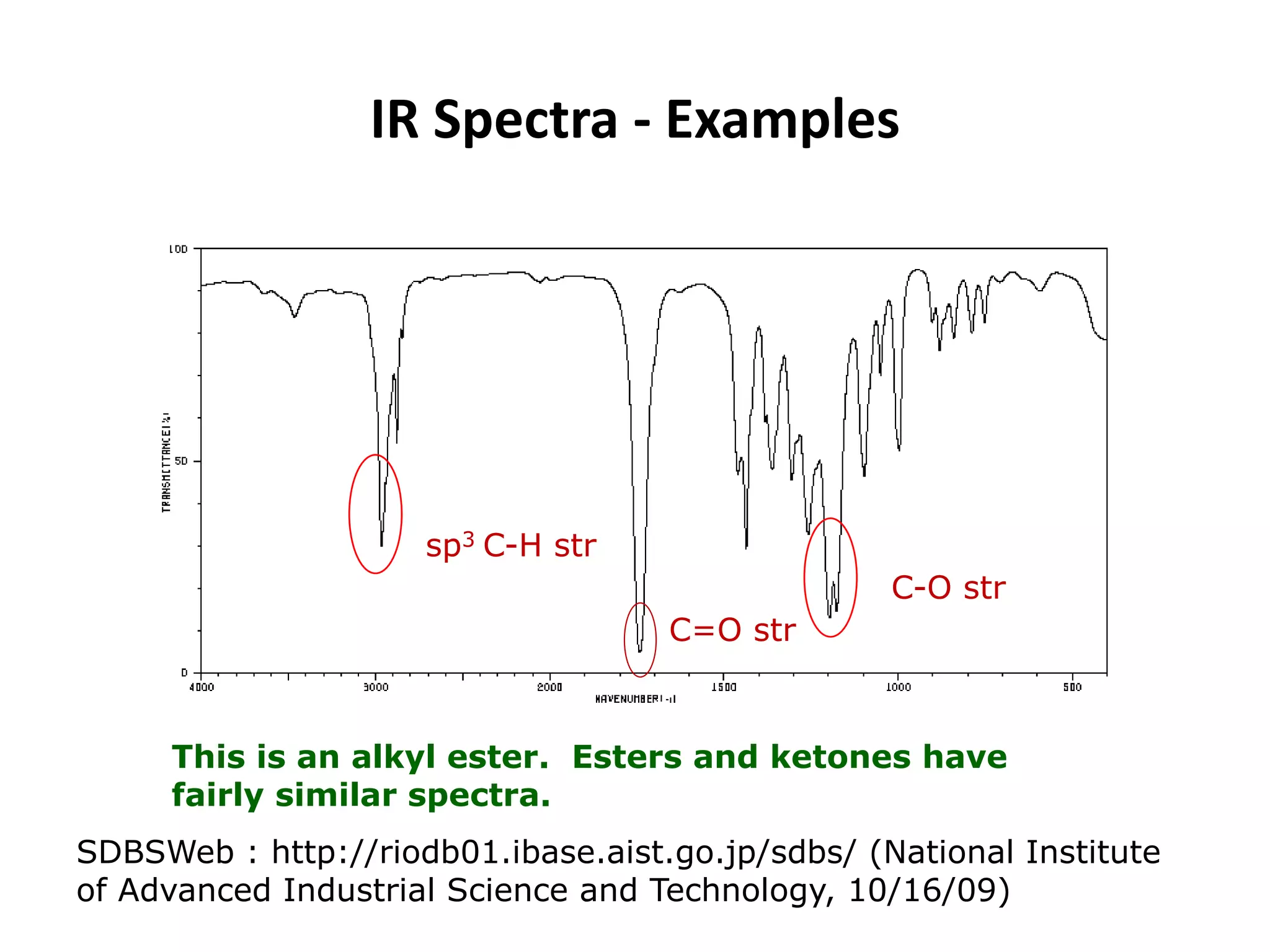
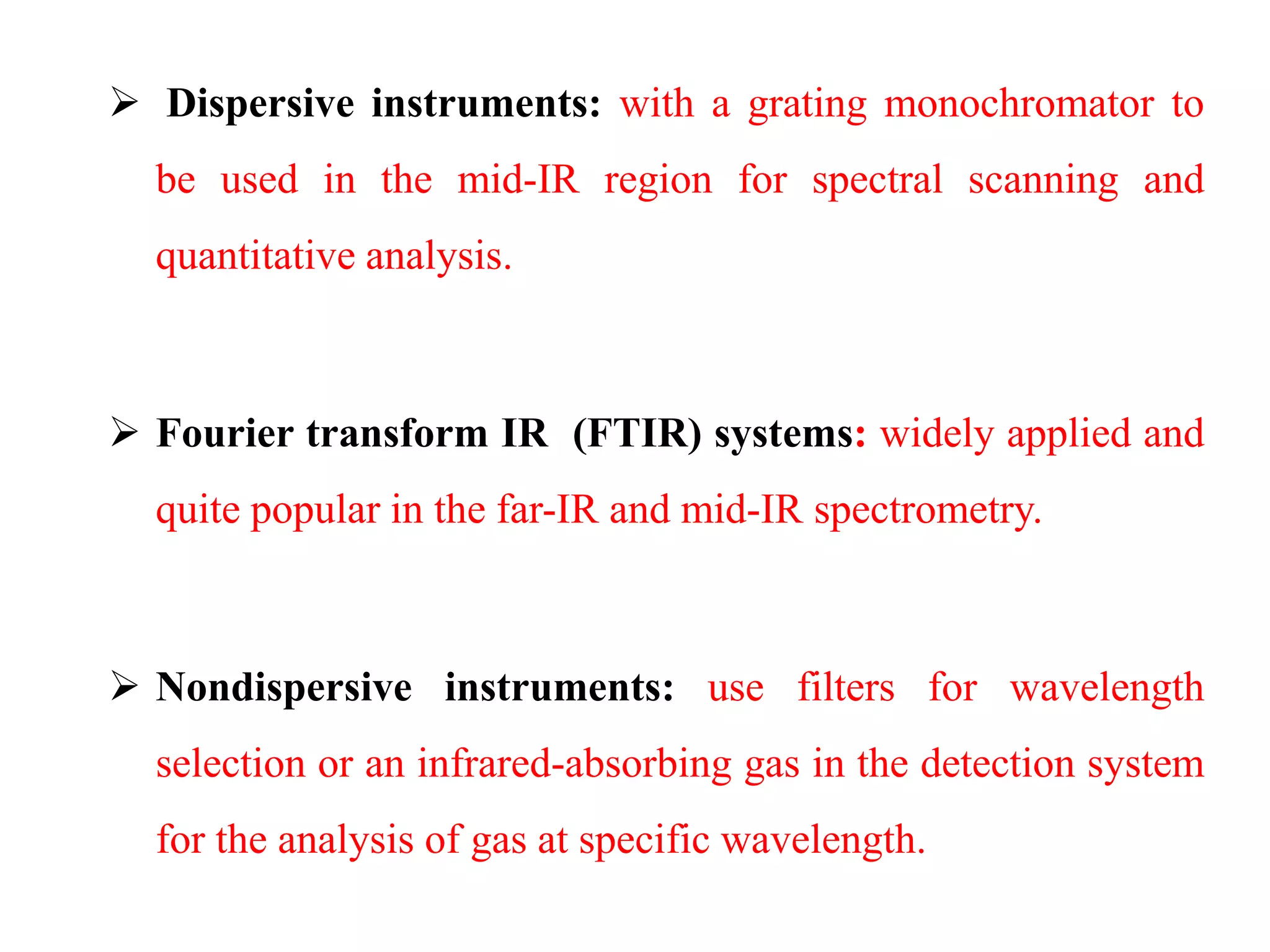
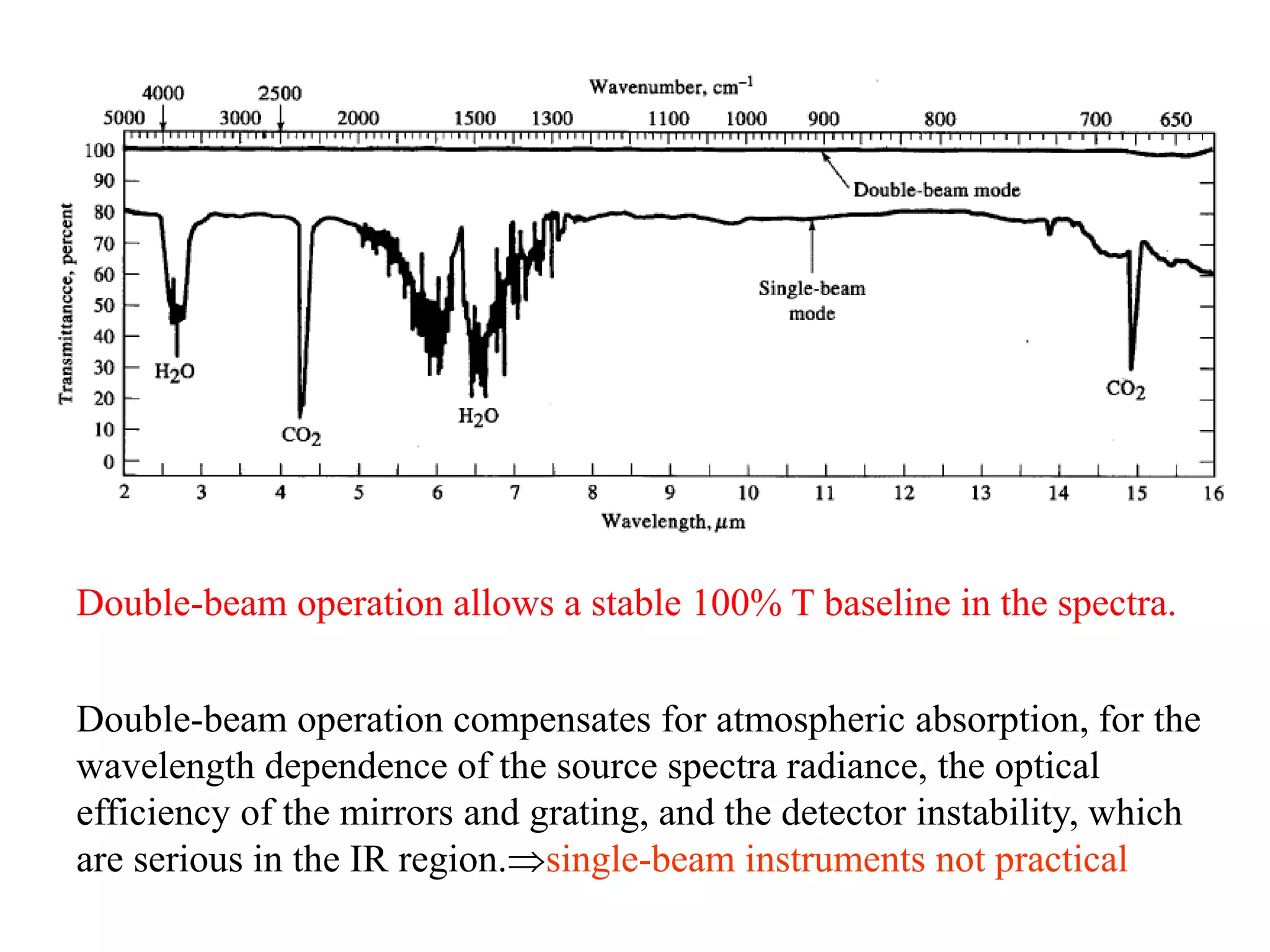
This document discusses the principles and techniques of Fourier Transform Infrared Spectroscopy (FTIR), focusing on the effects of vibrational coupling, overtone and combination bands, and strategies for interpreting infrared spectra. It provides guidance on analyzing specific functional groups based on their characteristic absorption frequencies and emphasizes the importance of considering factors like molecular symmetry and hydrogen bonding. Furthermore, it outlines the types of instrumentation used in FTIR, including dispersive and nondispersive instruments.