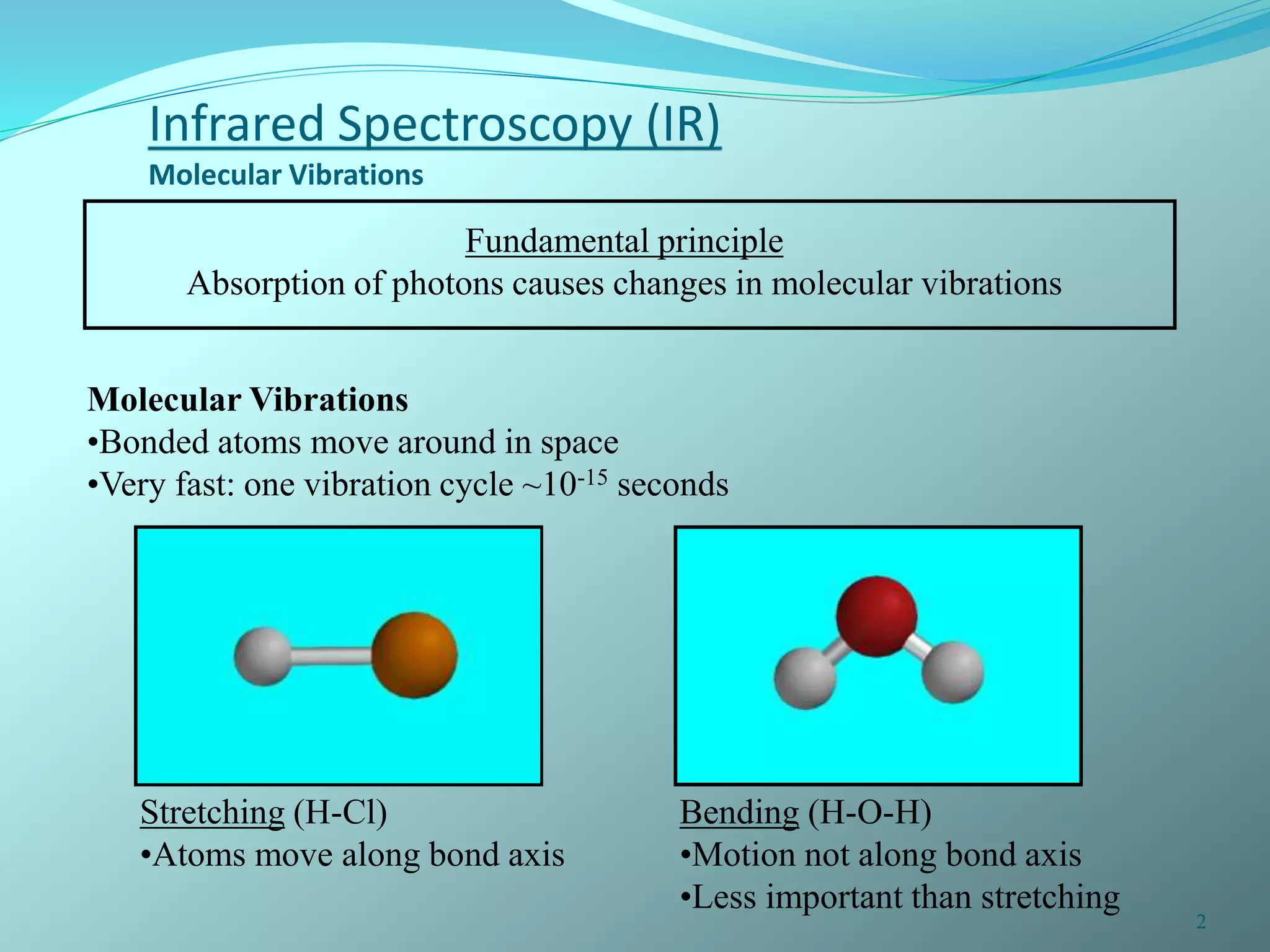
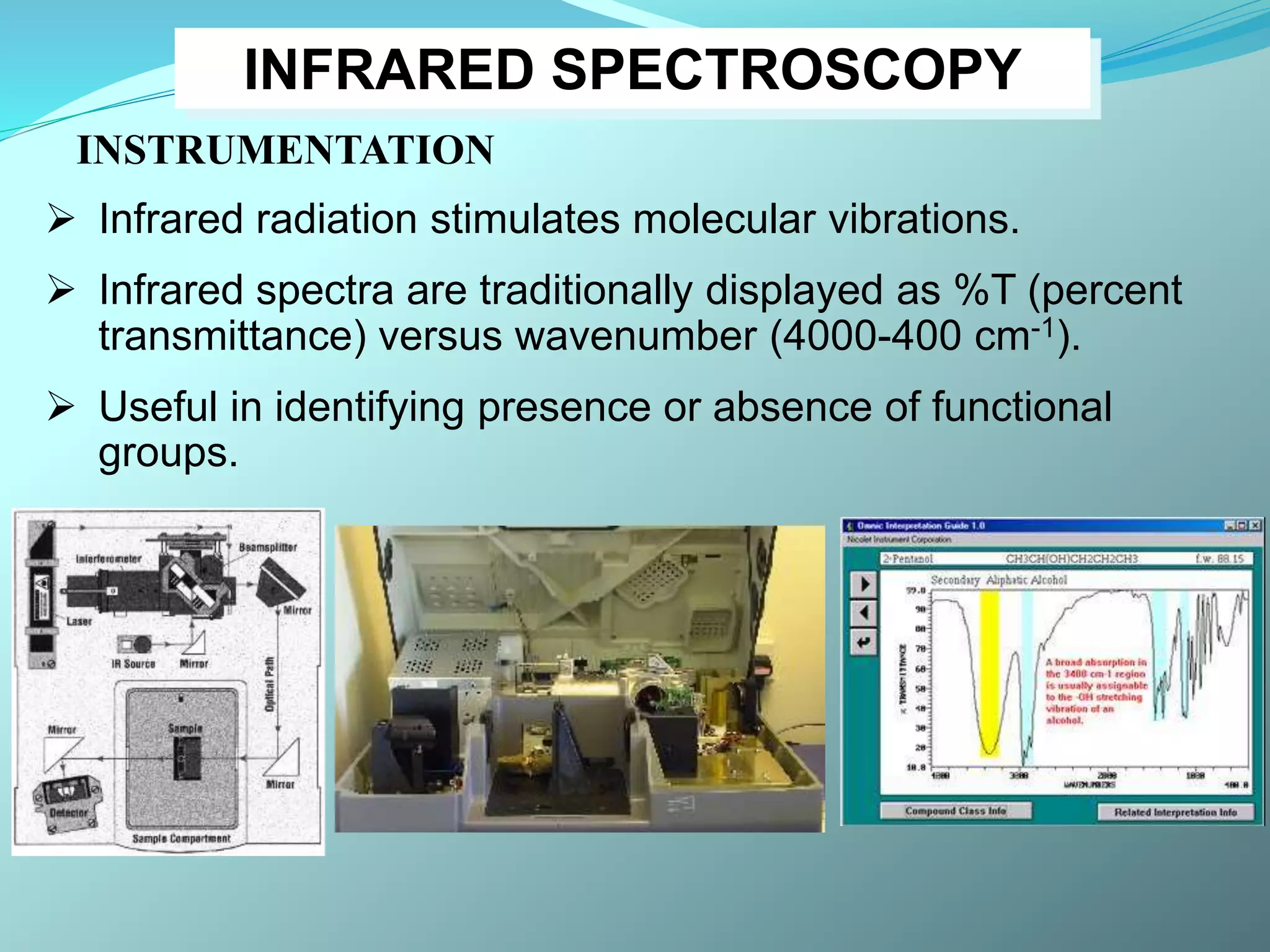
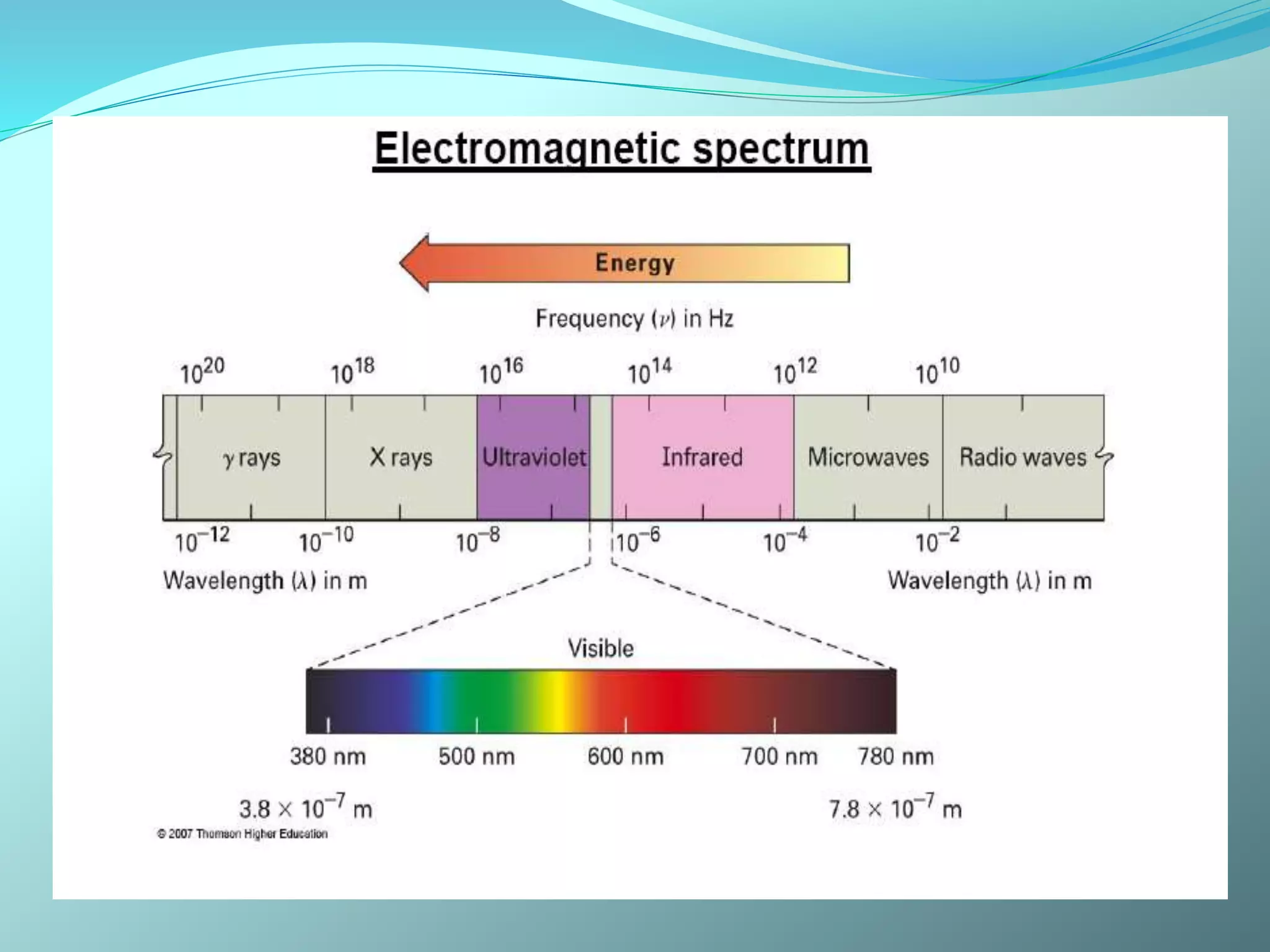
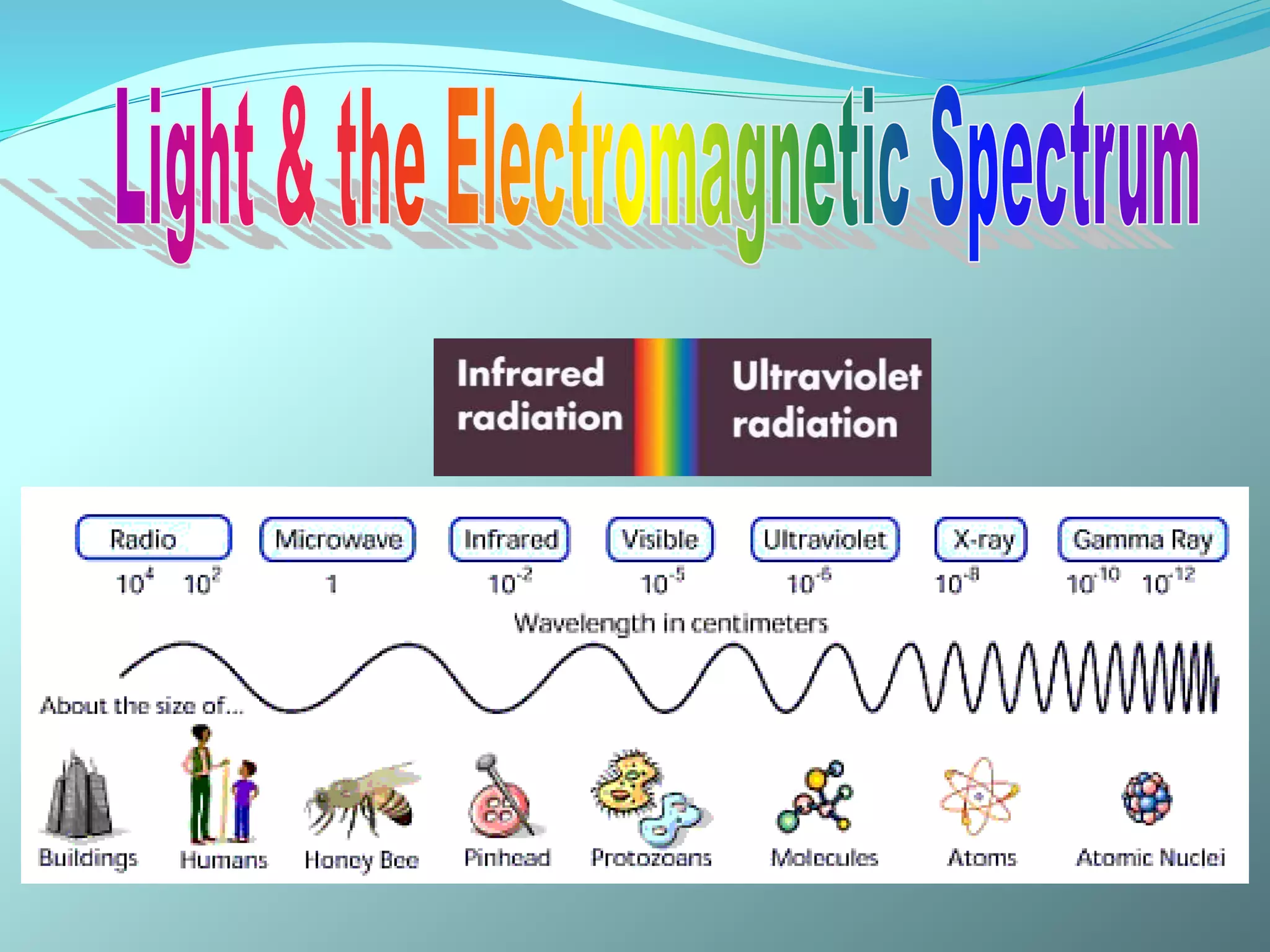
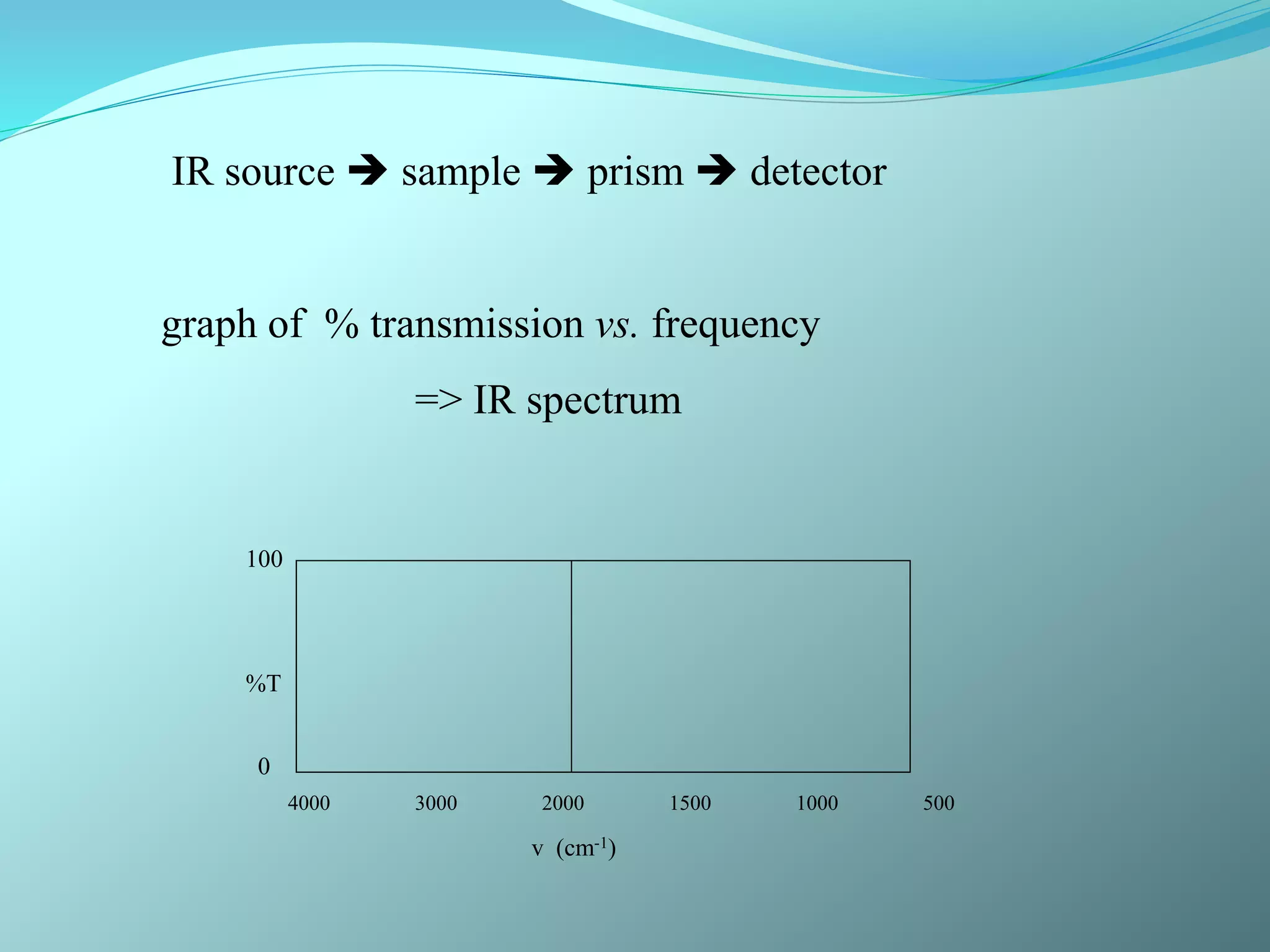
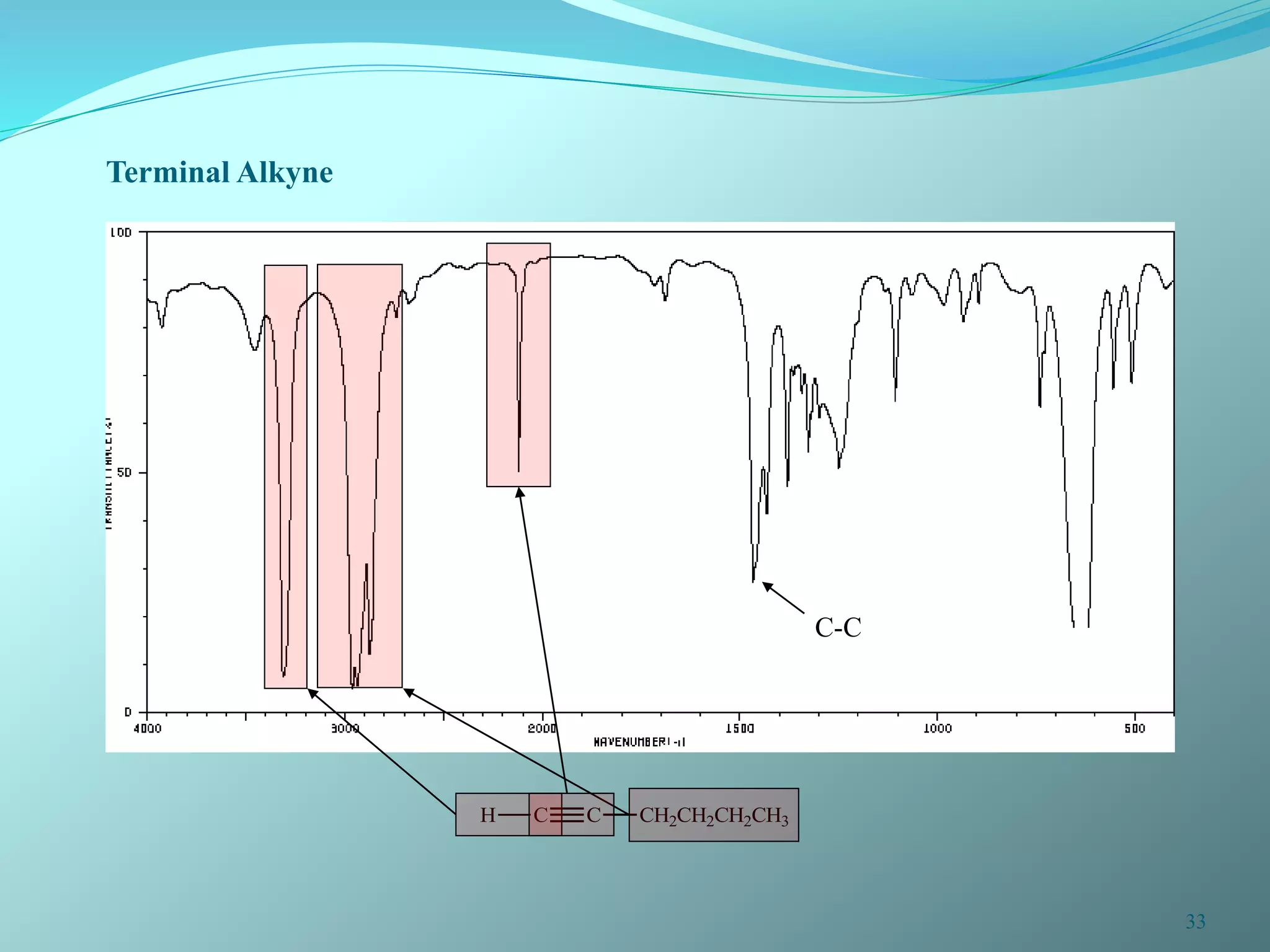
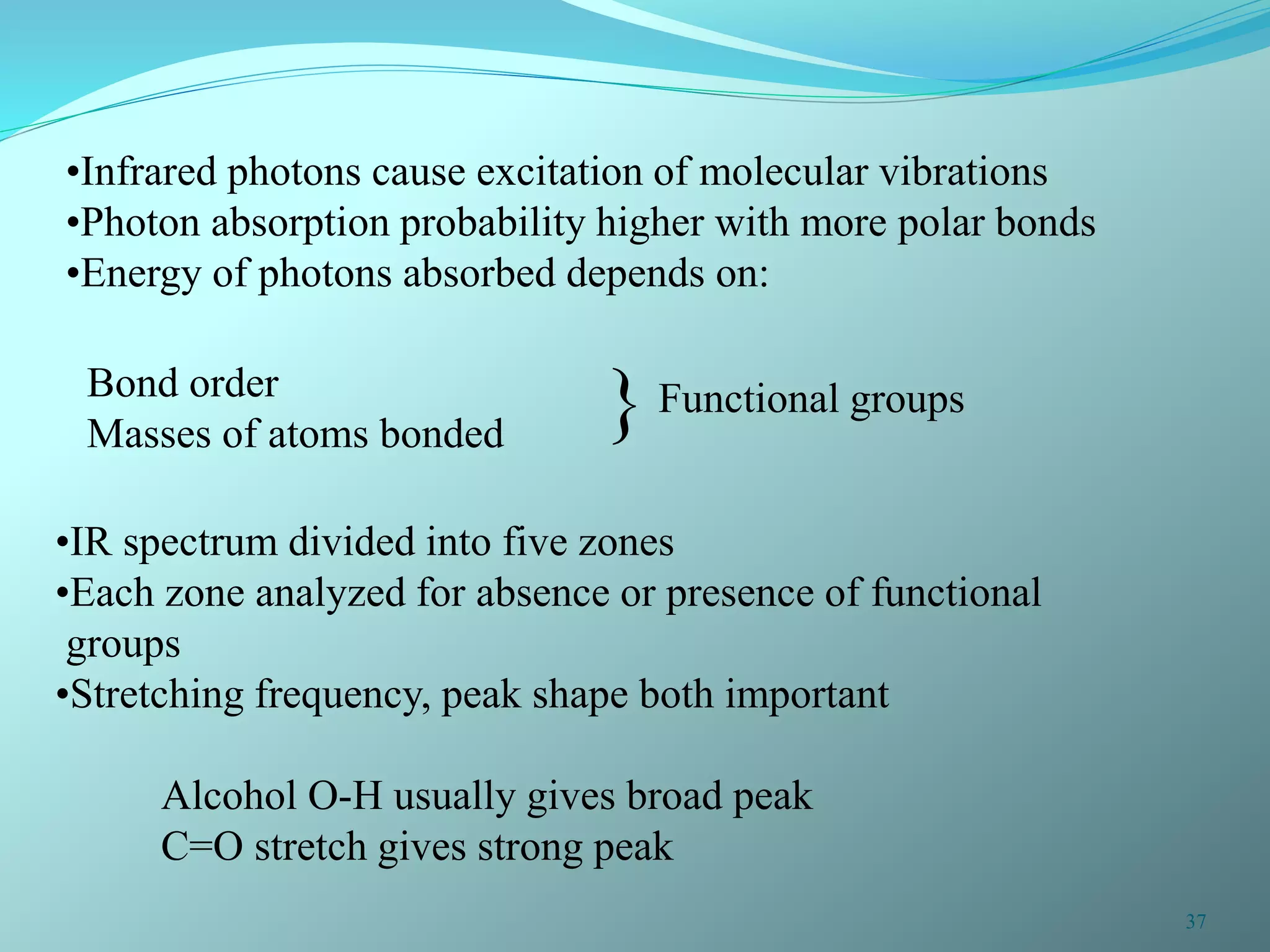
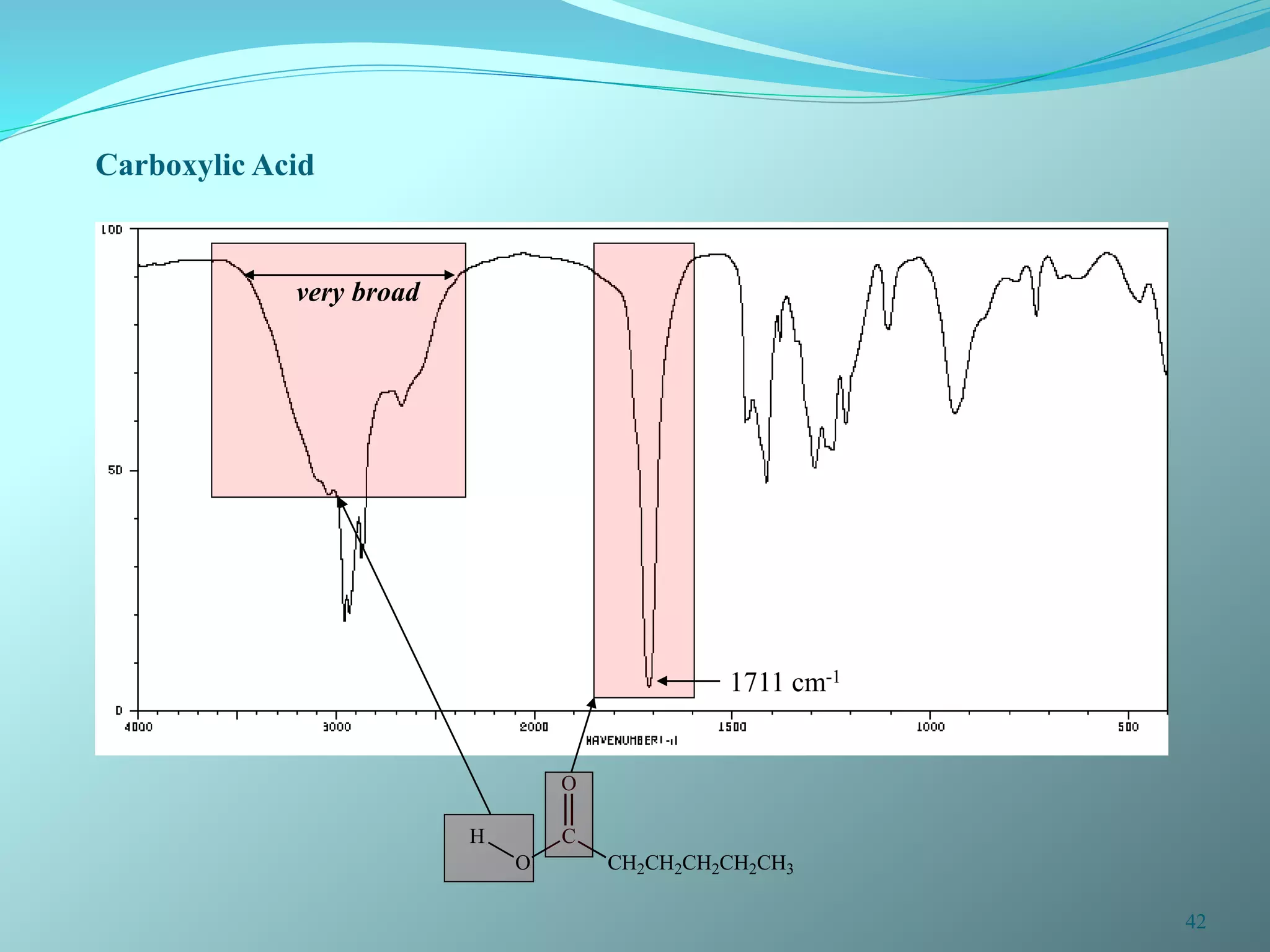
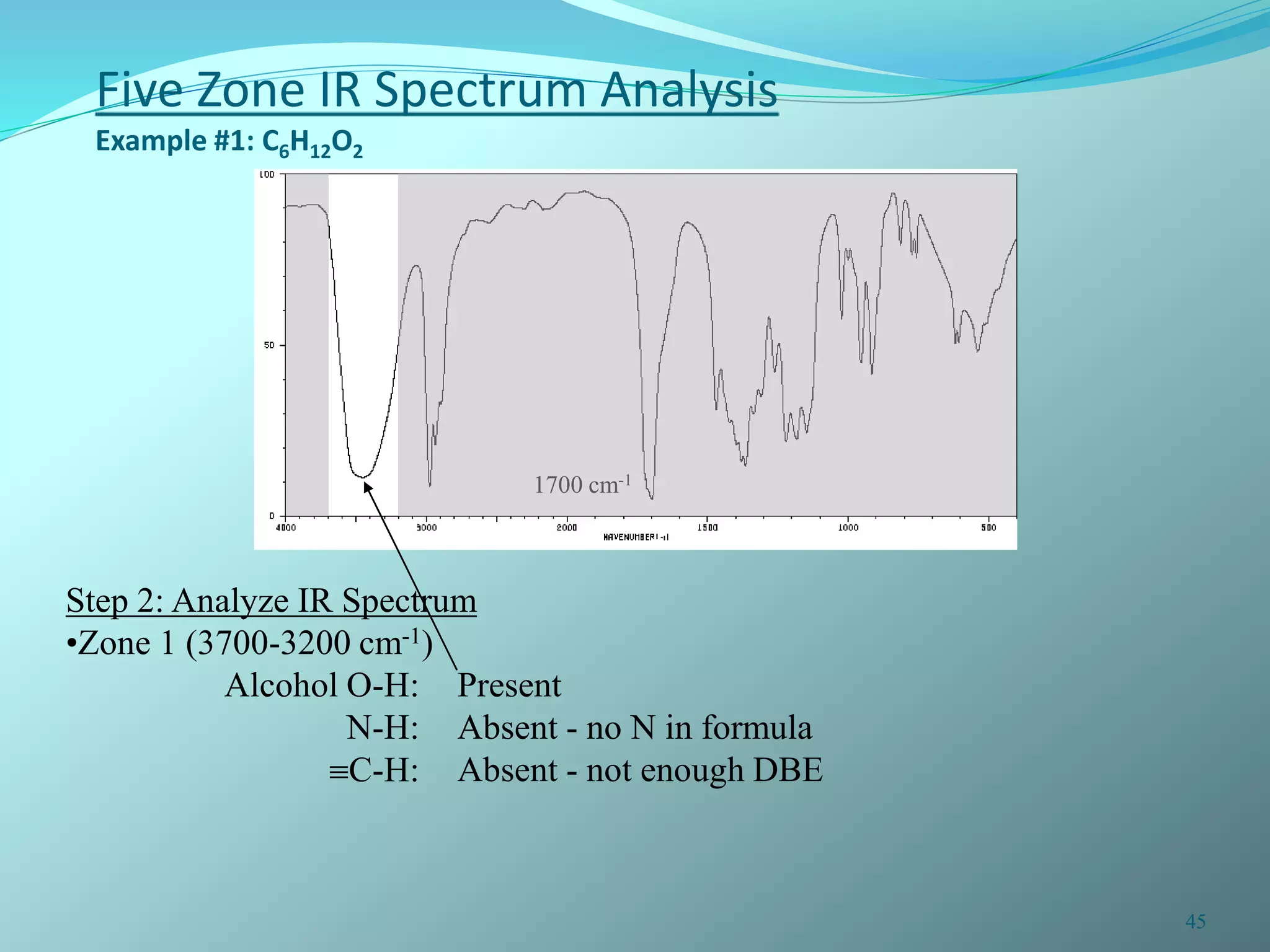
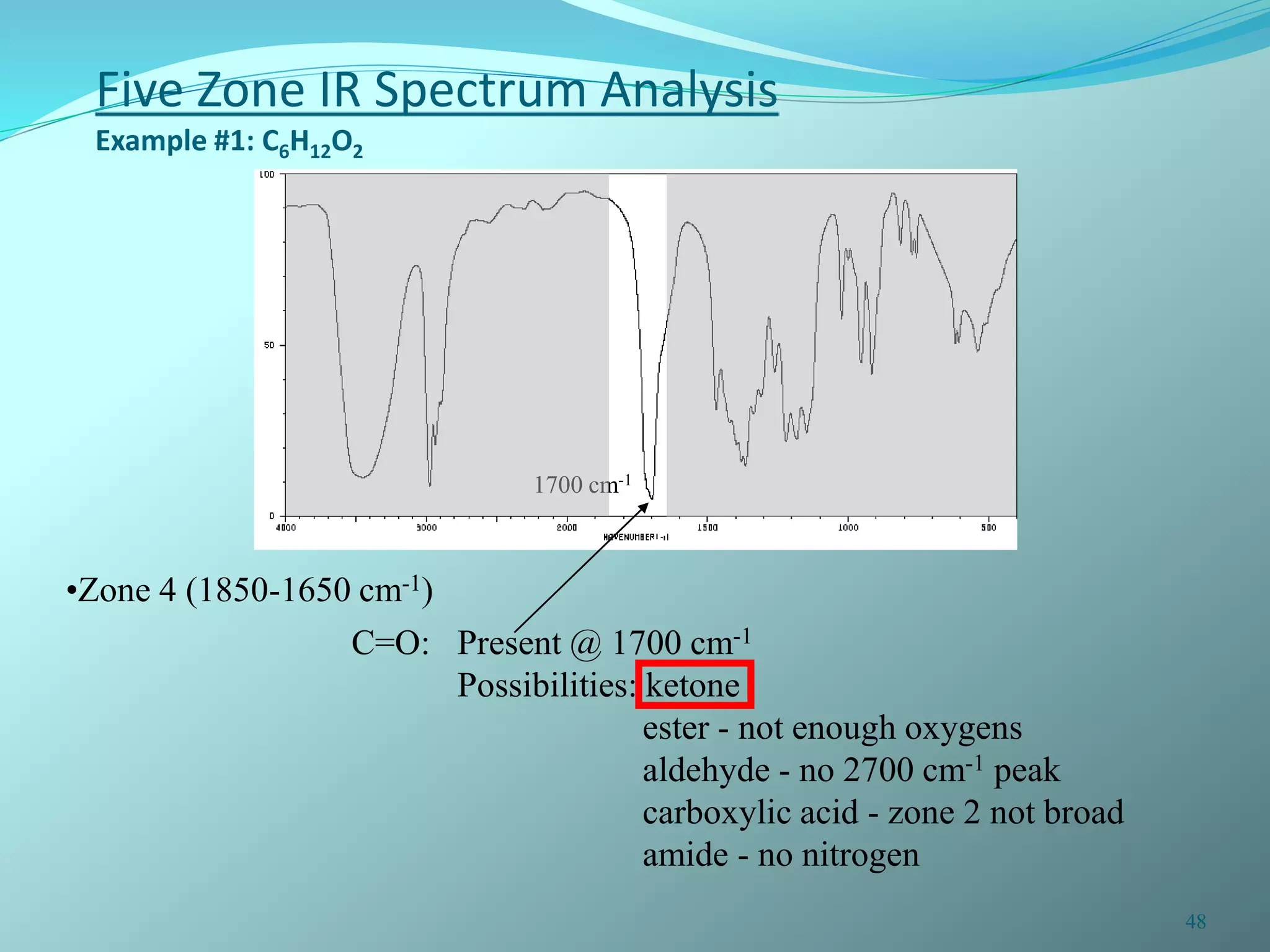
The document discusses infrared spectroscopy and molecular vibrations. It explains that infrared radiation causes changes in molecular vibrations by absorbing photons. It describes the different types of molecular vibrations including stretching and bending. It outlines the characteristic stretching frequencies of common functional groups in the five infrared regions. Examples are provided to demonstrate analyzing infrared spectra to determine molecular structures.