Embed presentation
Download to read offline





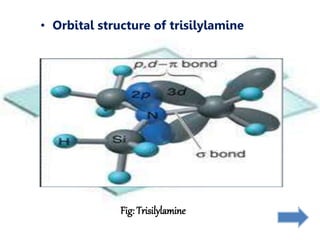
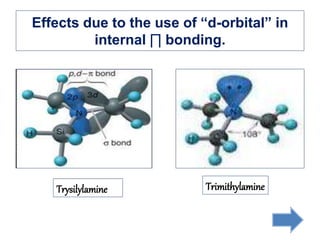
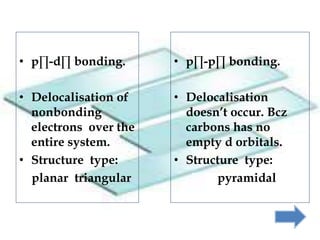
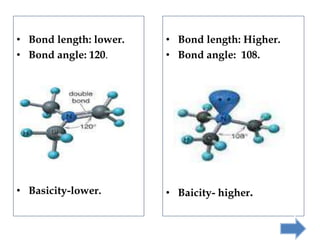

Khondaker Afrina Hoque presented on the use of d orbitals in internal π bonding in silylamines. Silylamines contain a silicon atom bound to a nitrogen atom. The presentation discussed the orbital structures of nitrogen, silicon, and trisilylamine. The use of d orbitals in trisilylamine allows for pπ-dπ bonding, delocalization of nonbonding electrons over the entire system, and a planar triangular structure, resulting in shorter bond lengths, 120 degree bond angles, and lower basicity compared to trimethylamine which forms pπ-pπ bonds without d orbital involvement.









