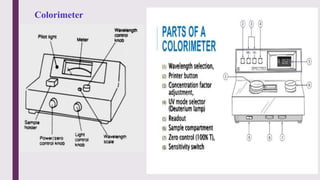
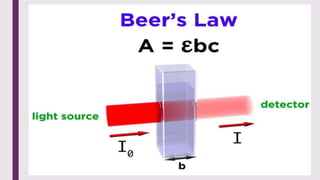
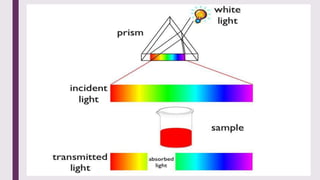
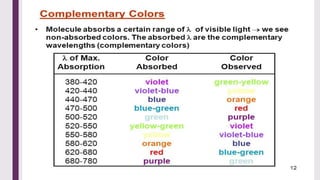
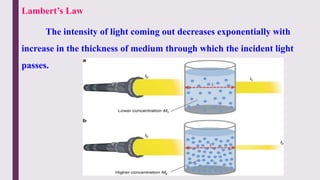
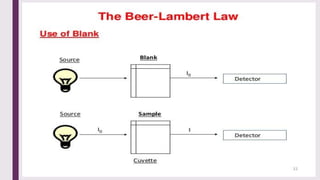
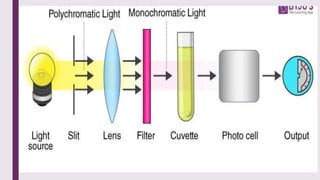
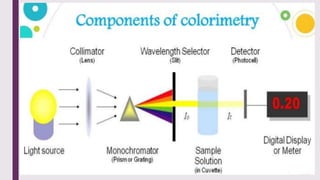
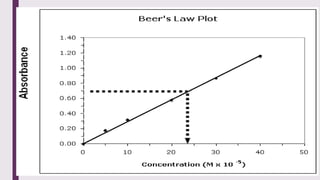
The document explains colorimetry, the technique of measuring colored substances in solution using a colorimeter. It describes the instrument's components, operational principles based on Beer’s and Lambert’s laws, and the procedure for measuring absorbance of solutions. Colorimeters are utilized in hospitals and laboratories for biochemical estimations and in various industries like food, paints, and textiles.