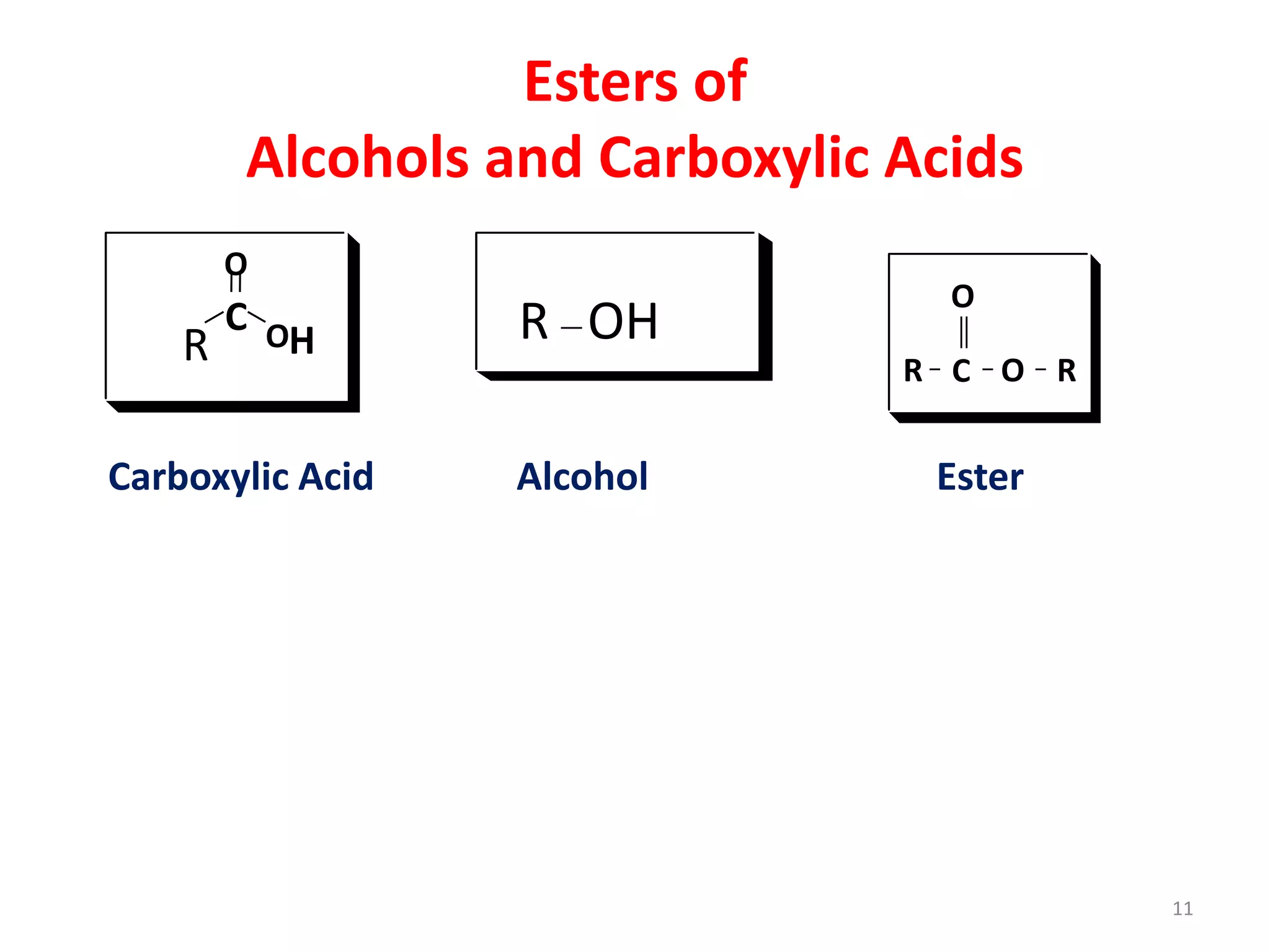
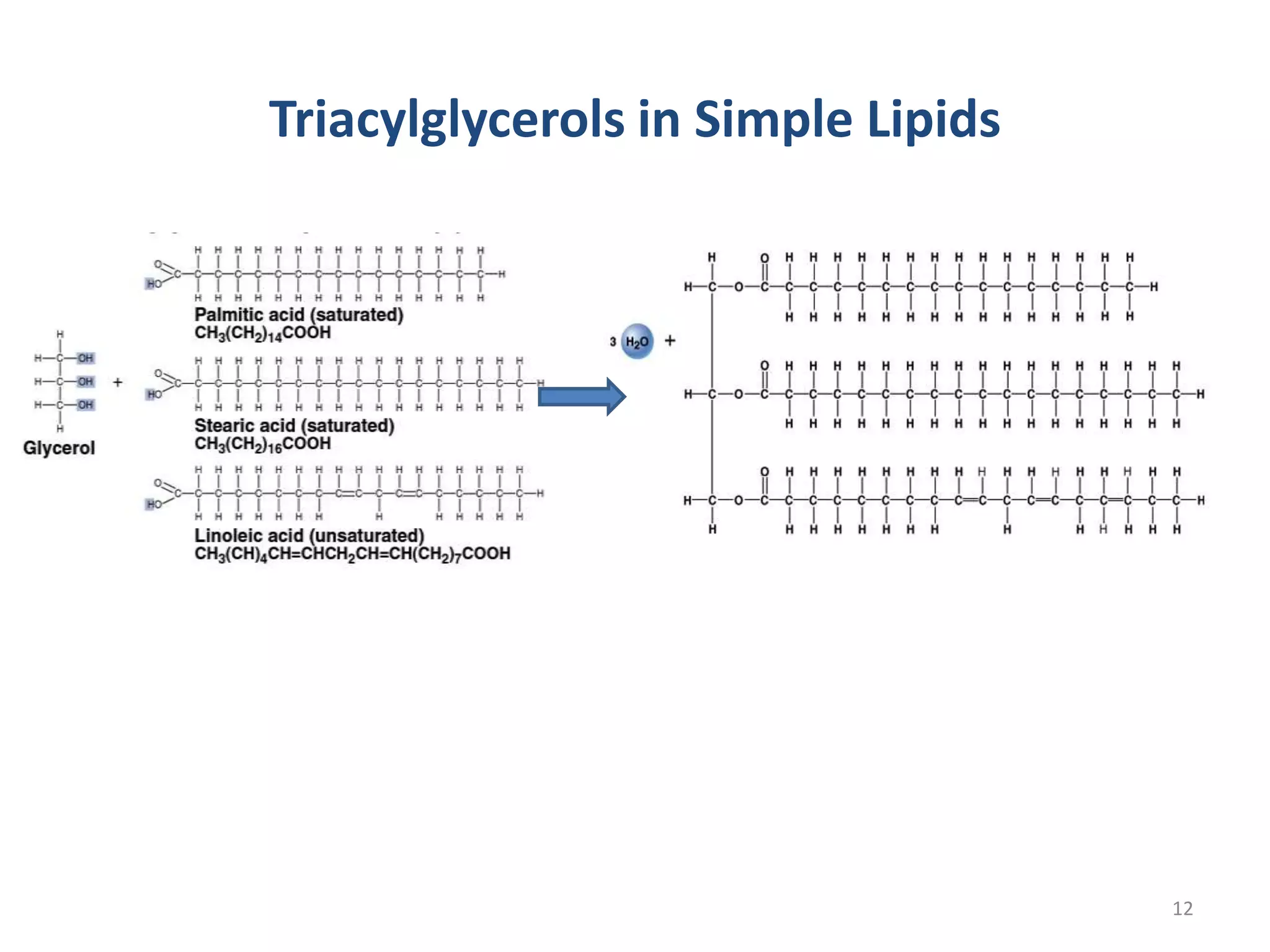
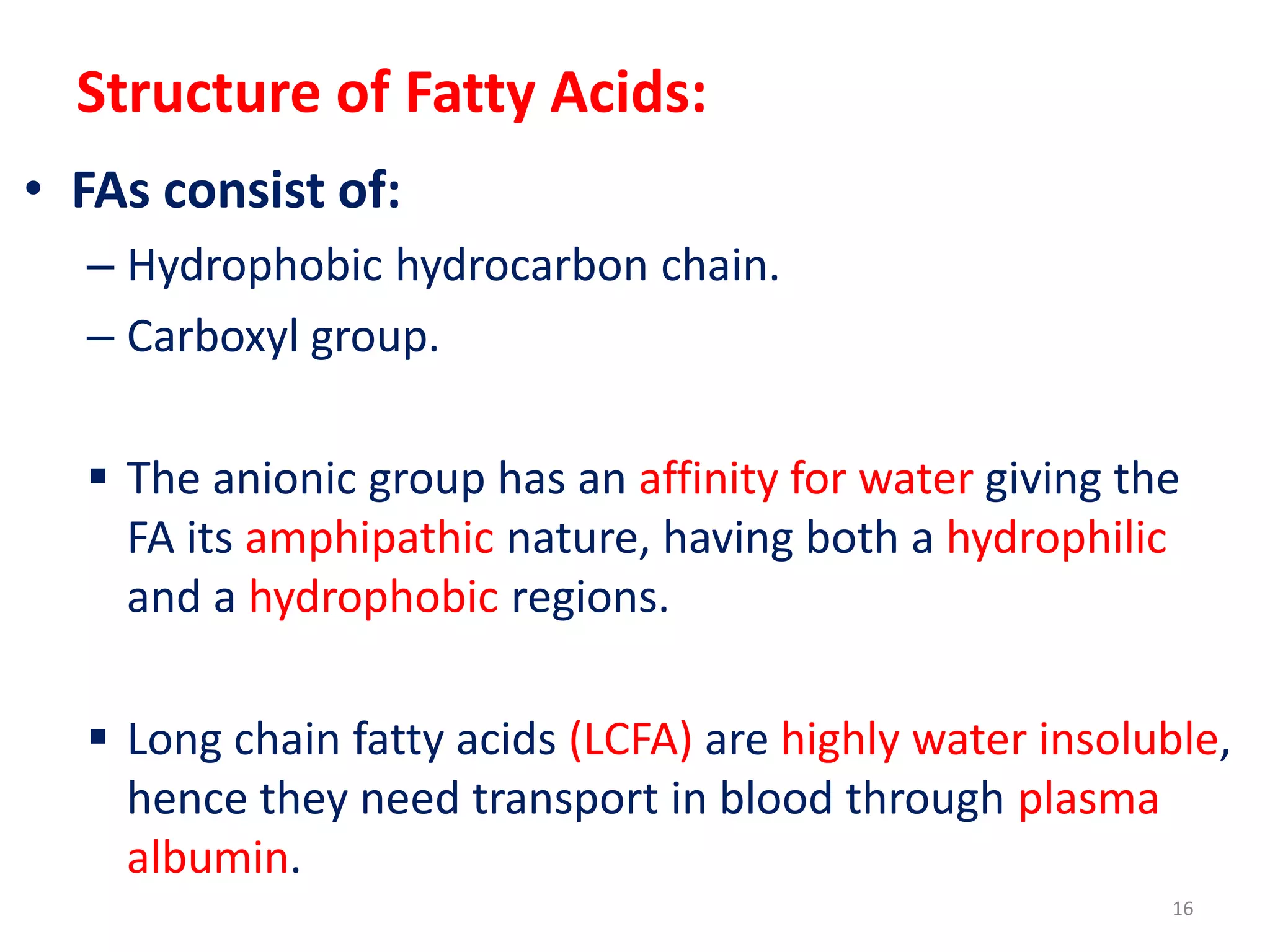
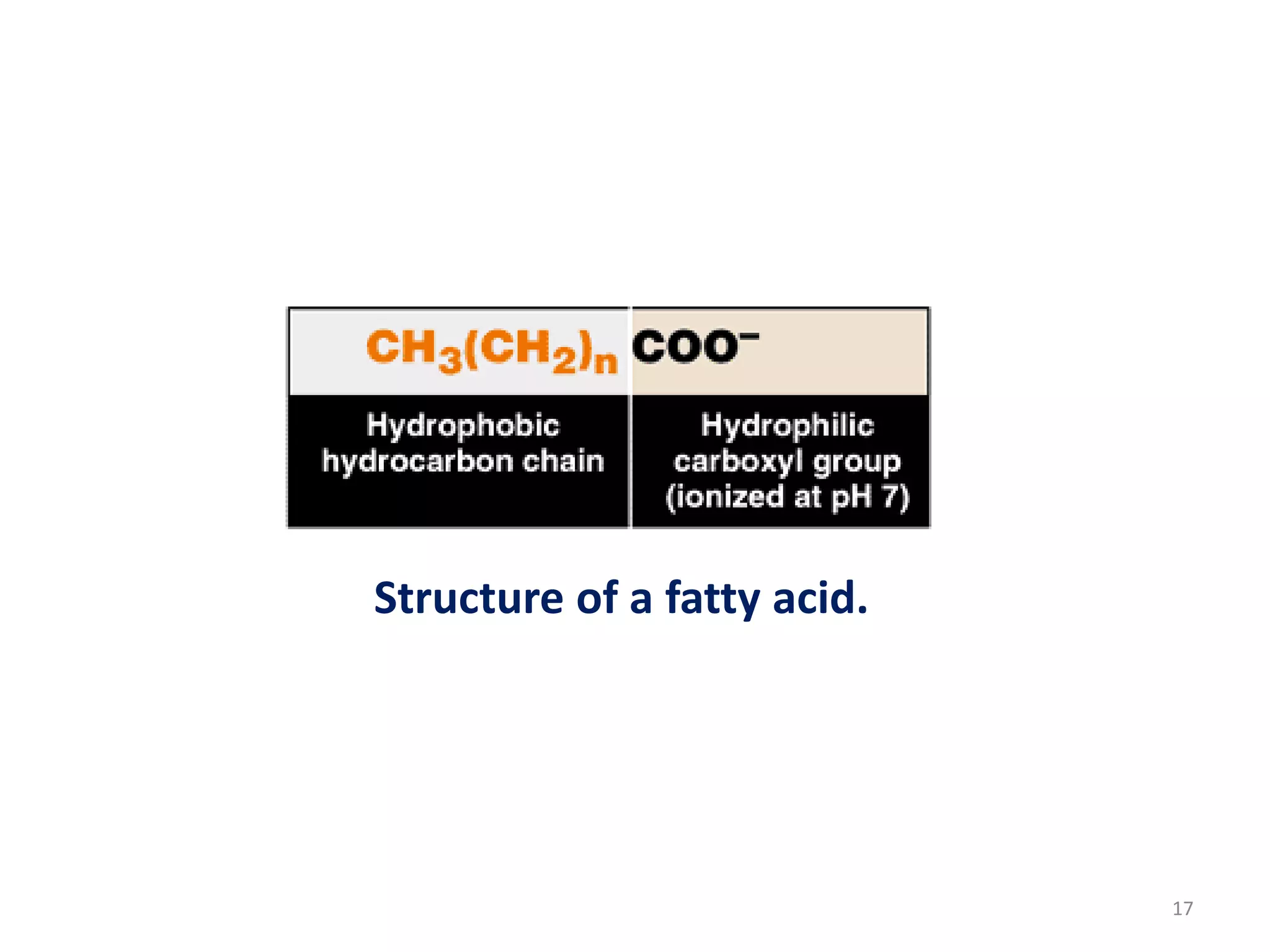
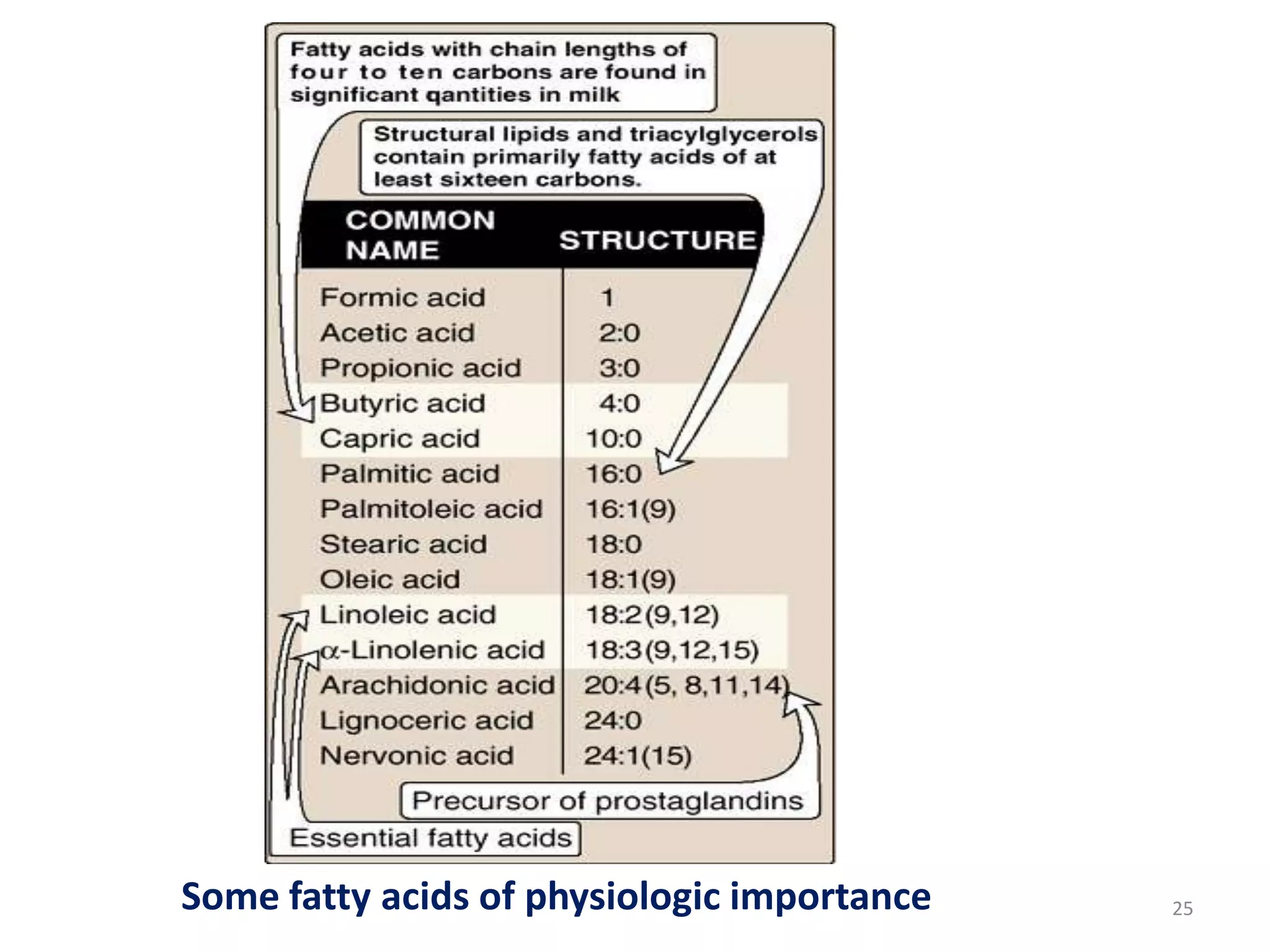
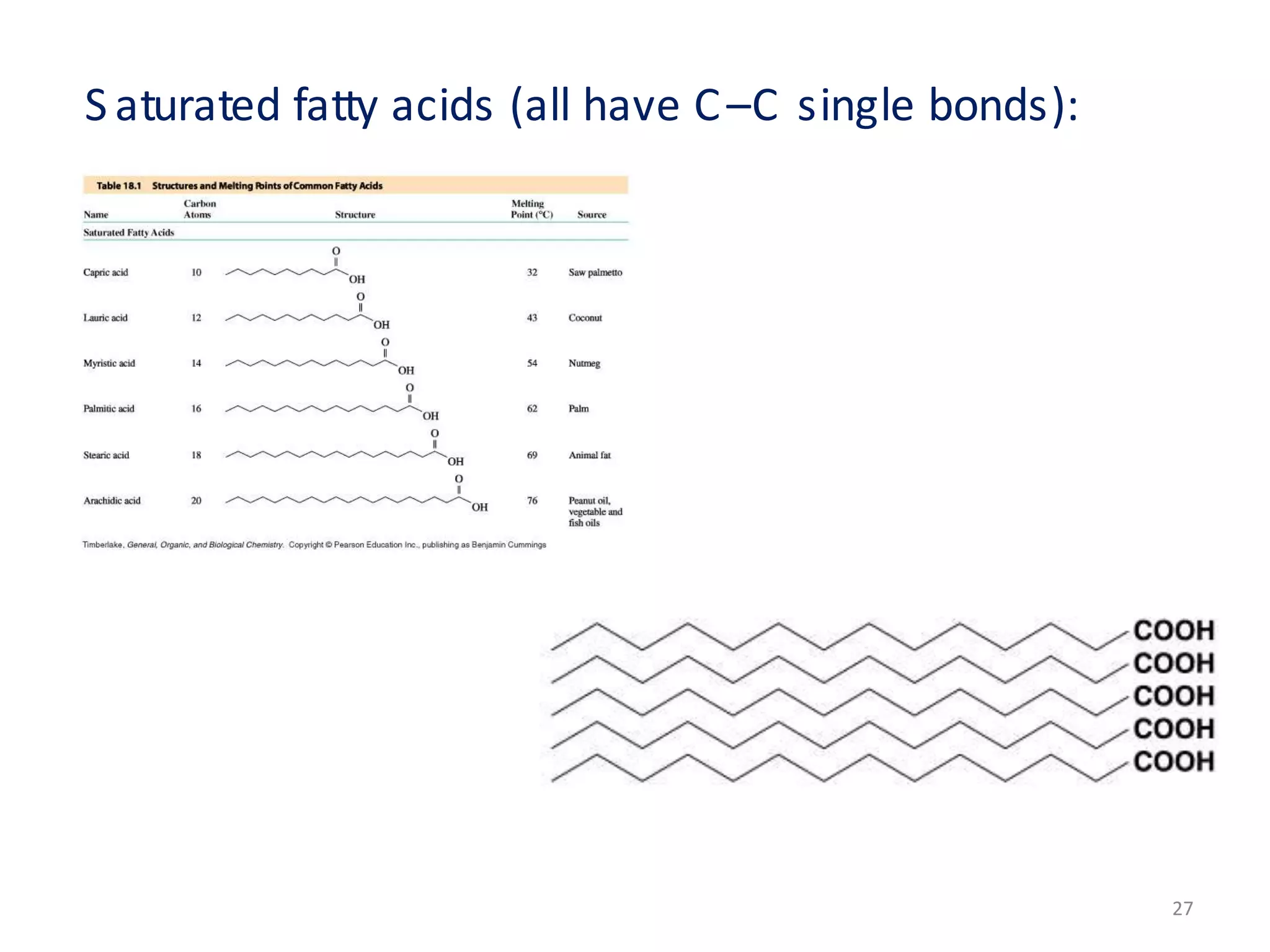
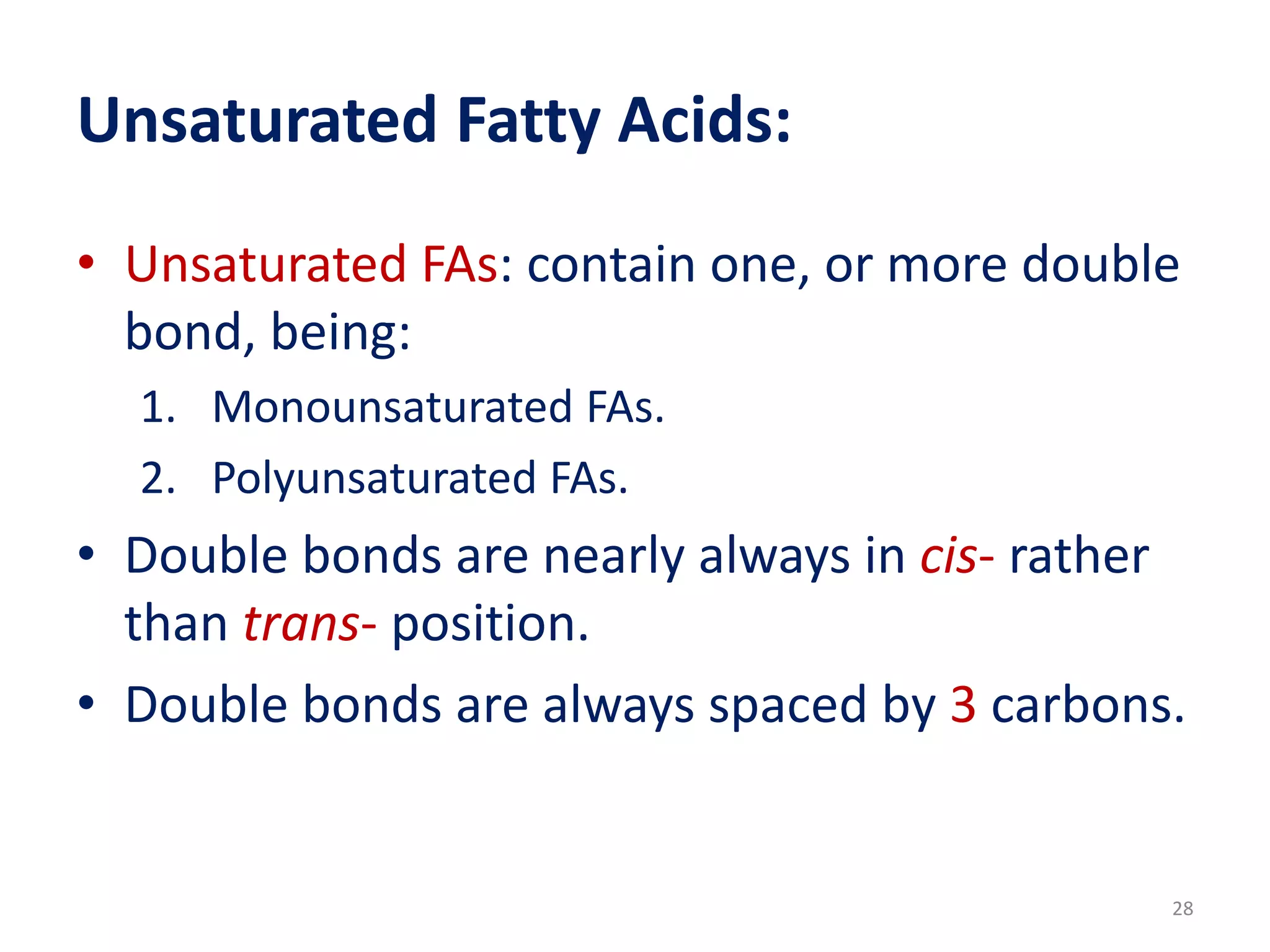
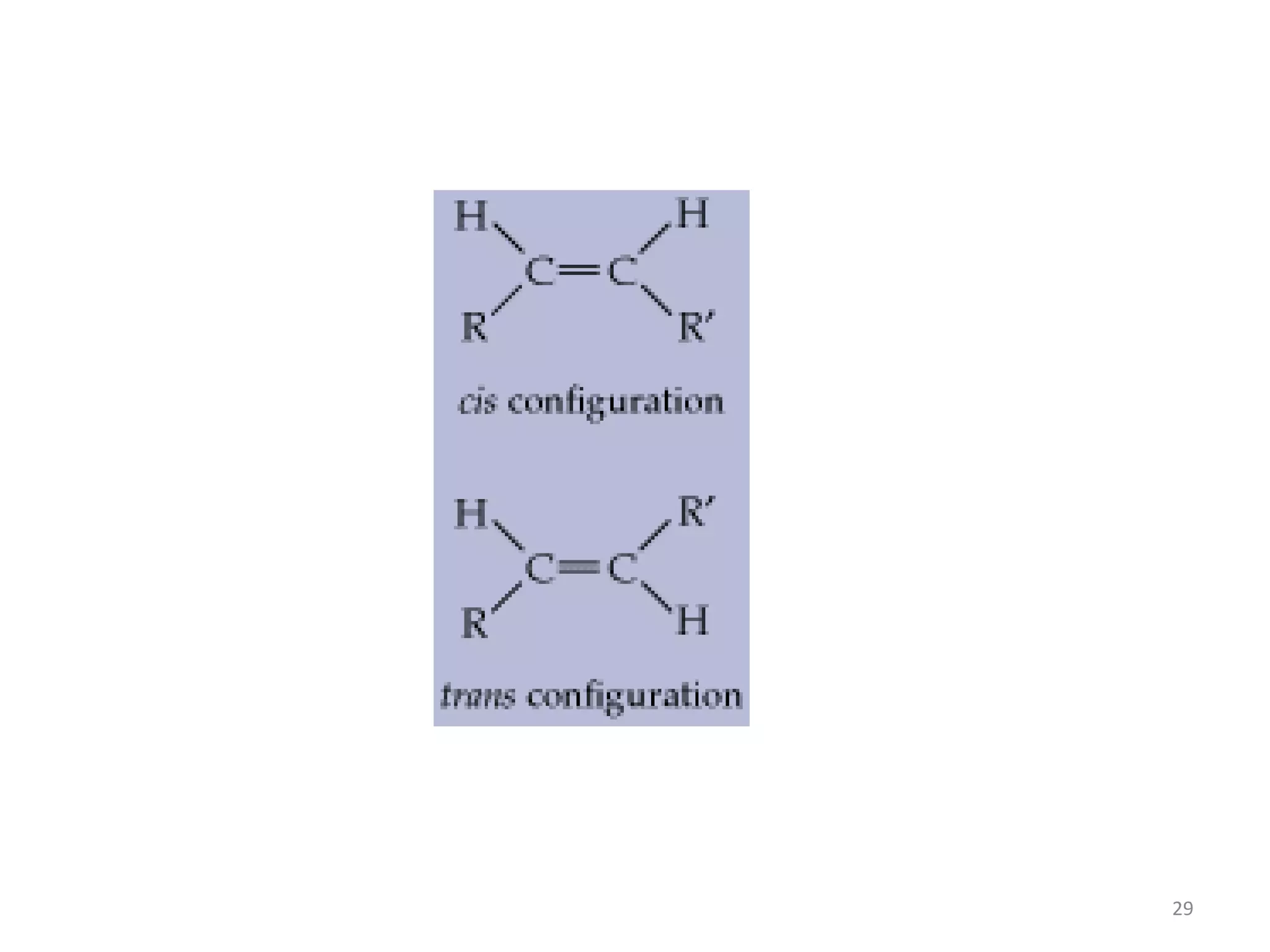
This document discusses simple lipids, including their classification and properties. It notes that simple lipids include fats, oils, and waxes, which are esters of fatty acids with alcohols like glycerol or high molecular weight alcohols. Key points covered include:
- Lipids are insoluble in water but soluble in nonpolar solvents. They serve important functions like energy storage, insulation, and as structural components of cell membranes.
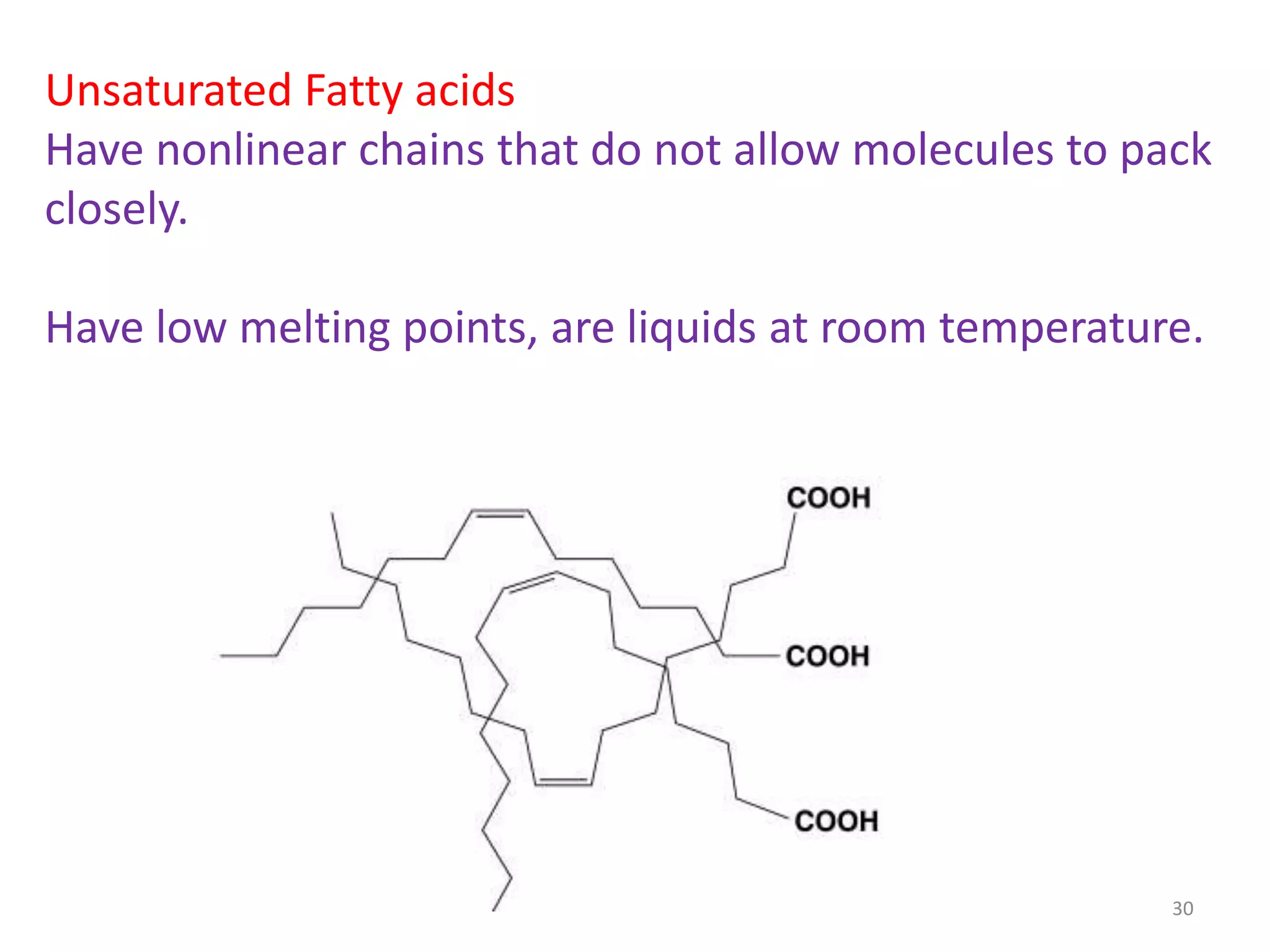
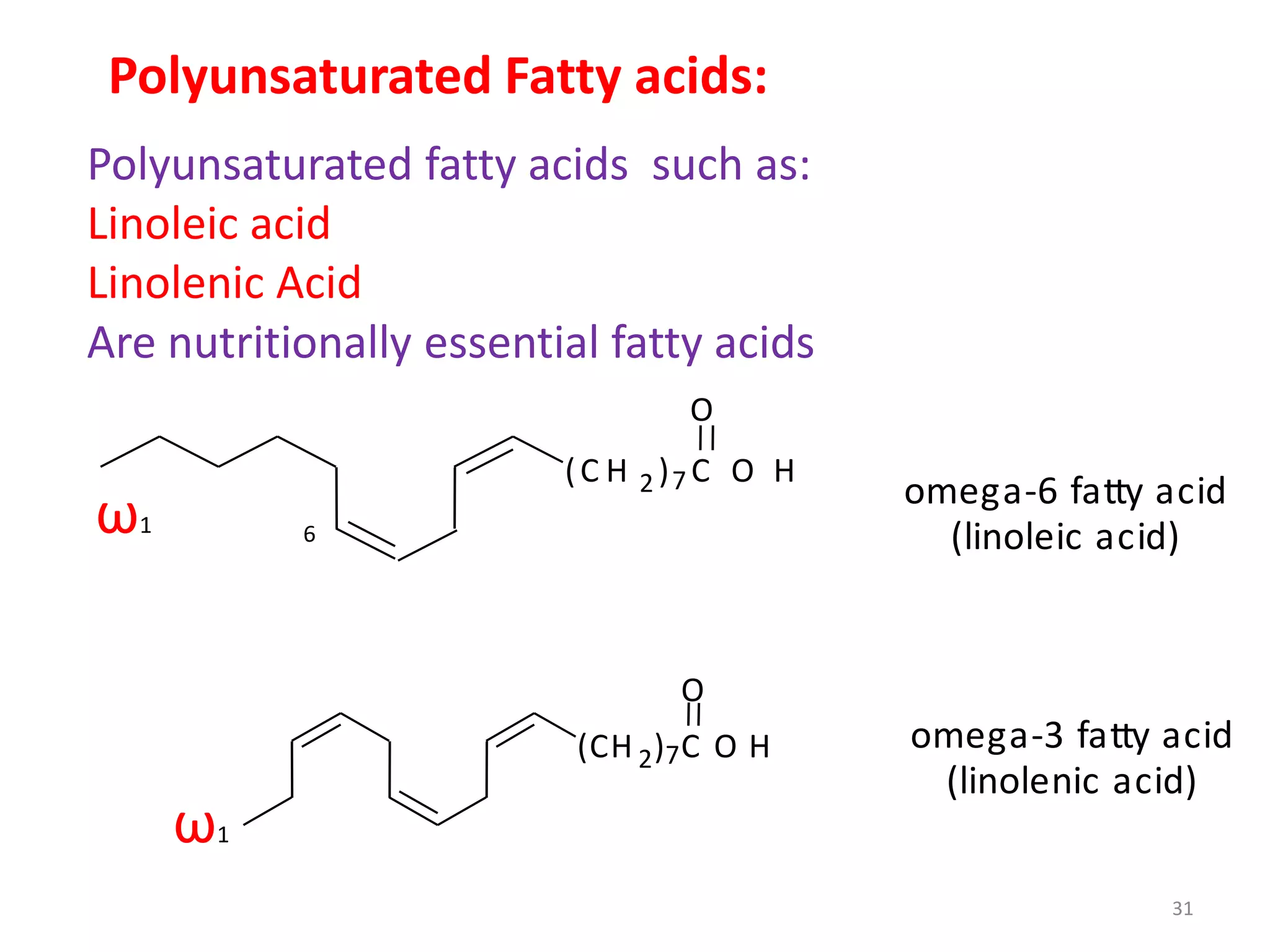
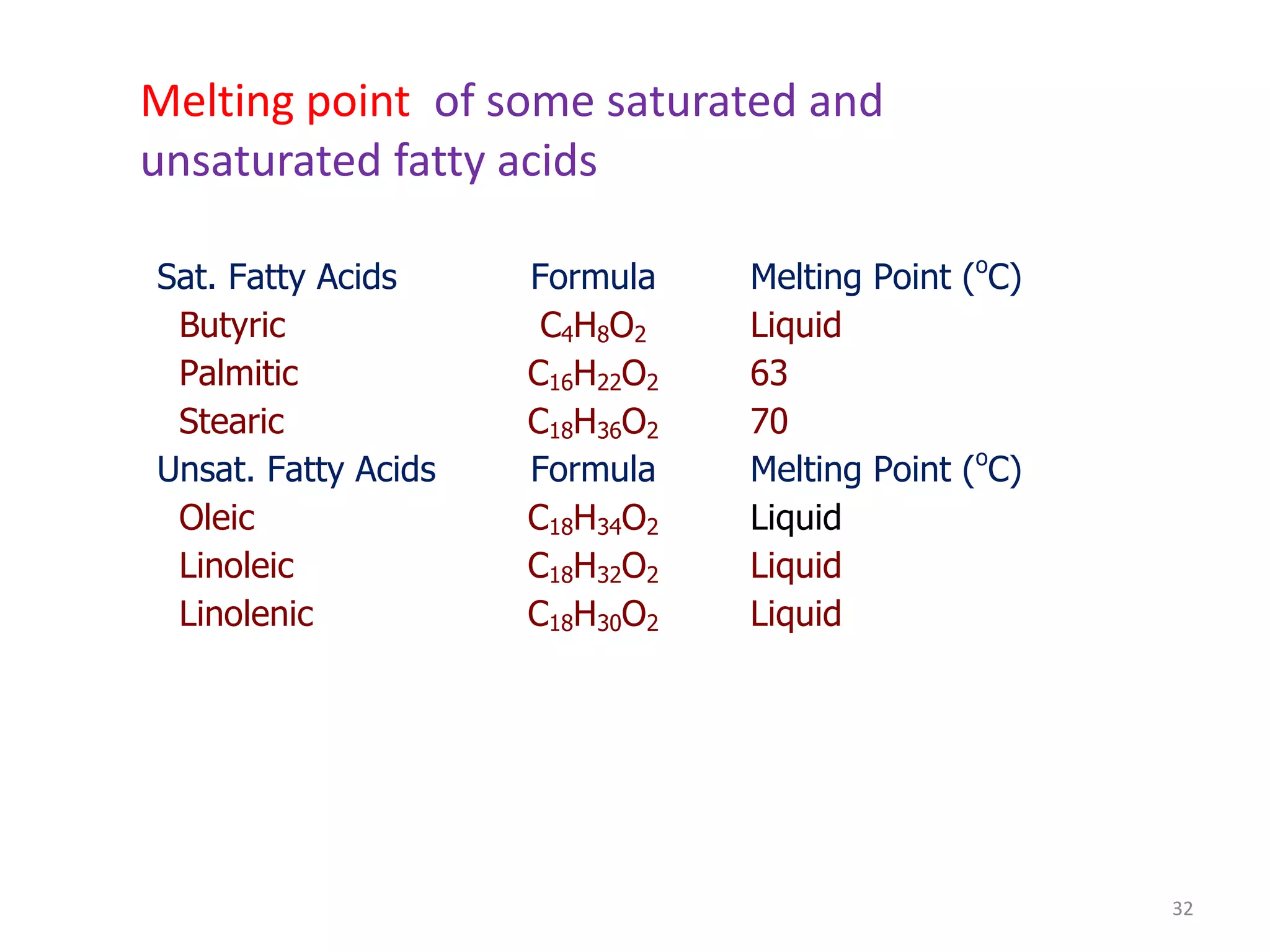
- Simple lipids specifically contain fatty acid esters of glycerol (fats and oils) or higher alcohols (waxes). They are classified as either saturated or unsaturated based on the number of double bonds in their fatty acid chains.

![• Lipids are a heterogamous group of chemical
compounds including:
• Fats
• Oils
• Waxes
• Steroids
• Other related compounds
[they are related more by their physical
properties than their chemical properties.]
2](https://image.slidesharecdn.com/simplelipids-130526203116-phpapp01/75/Simple-lipids-2-2048.jpg)

![Lipids are important dietary constituents:
• High energy value.
• Essential fatty acids.
• Fat soluble vitamins [ A D E K].
4](https://image.slidesharecdn.com/simplelipids-130526203116-phpapp01/75/Simple-lipids-4-2048.jpg)




![3. Precursor and derived lipids:
• This group includes:
–Fatty Acids.
–Glycerol.
–Cholesterol.
–Steroid hormones.
–Fatty aldehydes.
–Fat soluble vitamins [ A D E K].
–Some other alcohols.
10](https://image.slidesharecdn.com/simplelipids-130526203116-phpapp01/75/Simple-lipids-10-2048.jpg)