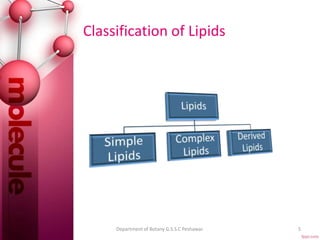
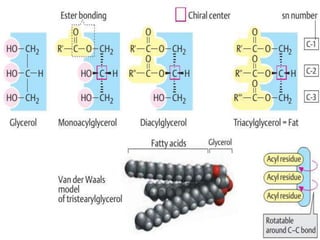
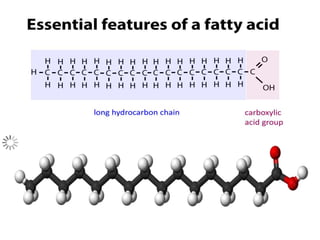
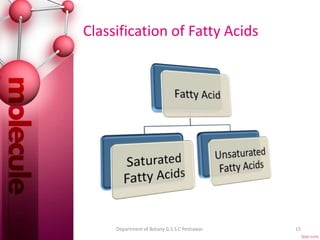
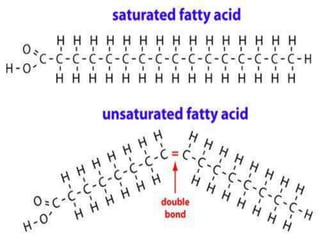
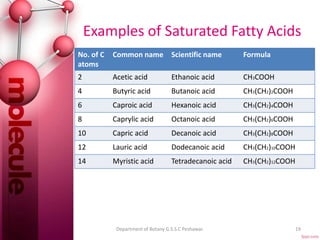
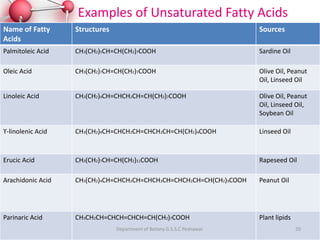
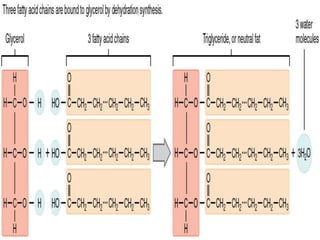
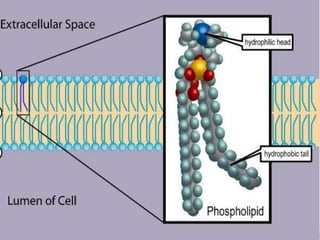
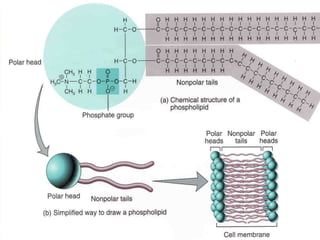
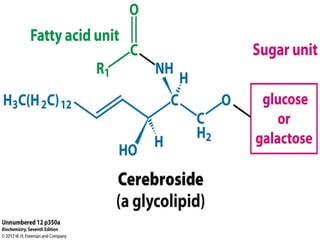
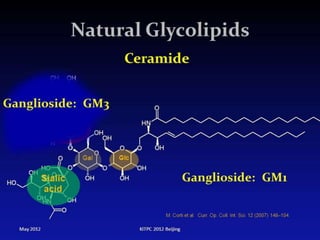
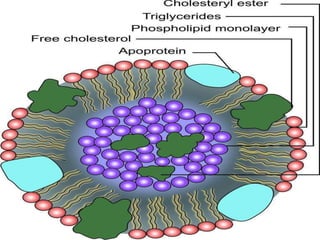
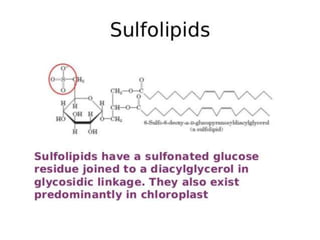
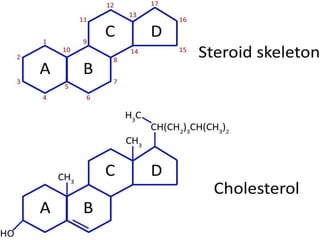
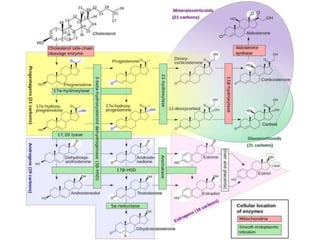
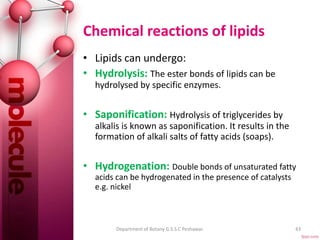
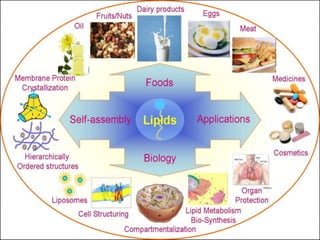
The document provides a comprehensive overview of lipids, detailing their classification into simple and complex lipids, and their chemical and functional properties. It discusses different types of fatty acids, including saturation levels, and the significance of triglycerides, phospholipids, and steroids in biological systems. Additionally, it covers the chemical reactions lipids undergo and their essential roles in energy storage, structural components of cell membranes, and metabolic regulation.