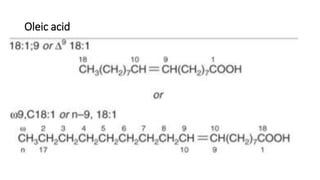
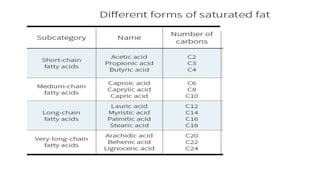
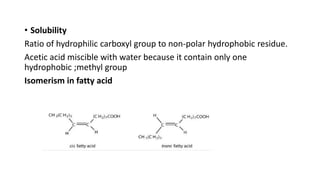
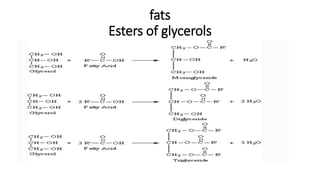
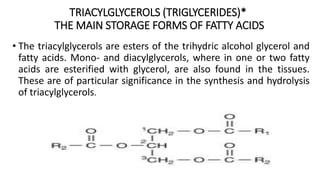
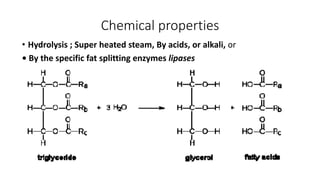
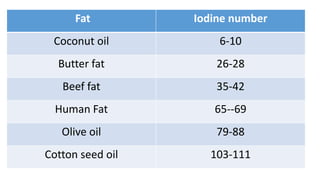
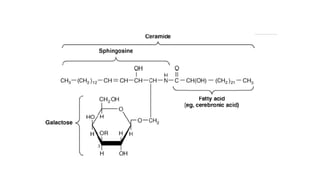
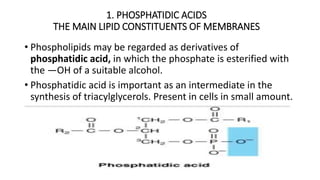
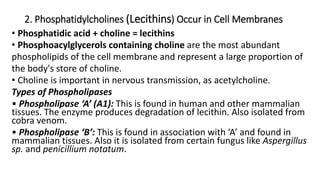
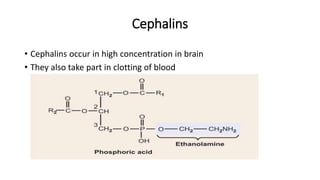
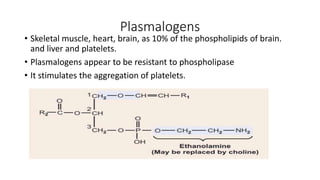
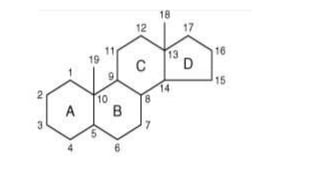
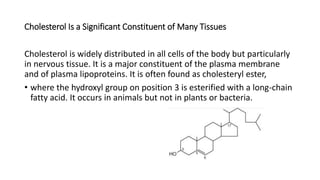
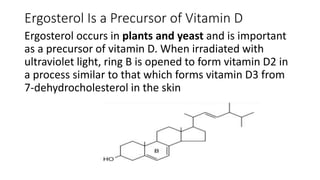
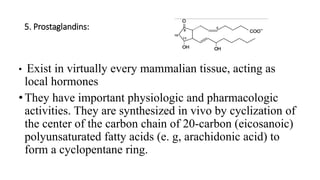
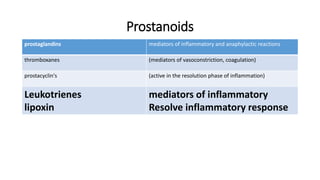
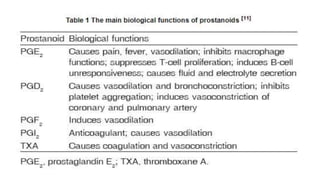
The document defines and describes various types of lipids. It discusses simple lipids like fats and waxes, as well as complex lipids including phospholipids, glycolipids, and gangliosides. Key points covered include the structures and functions of fatty acids and triglycerides, as well as the roles of lipids in energy storage, insulation, cell membranes, and lipid transport. Steroids such as cholesterol are also introduced.