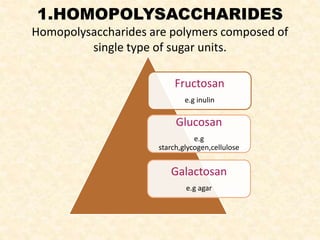
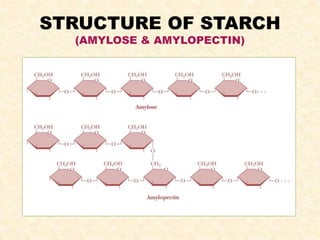
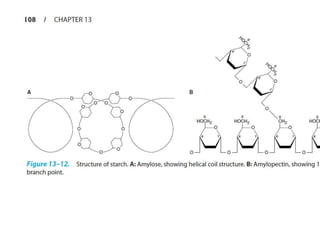
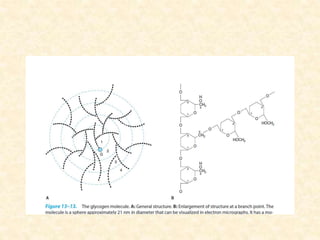
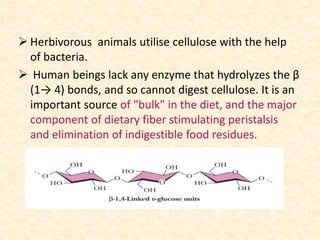
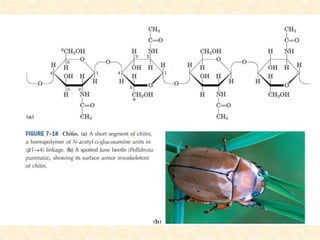
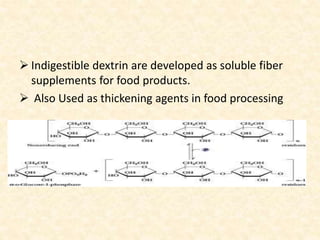
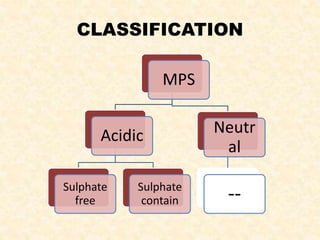
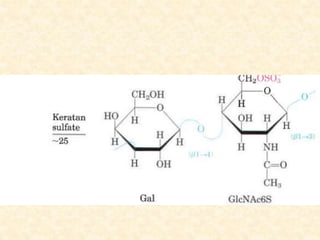
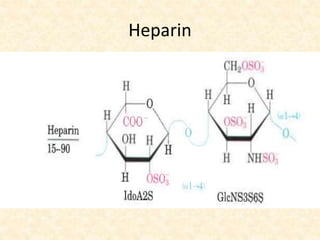
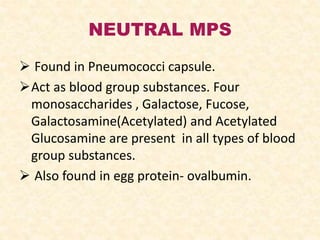
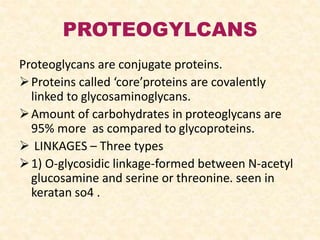
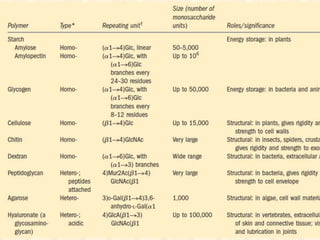
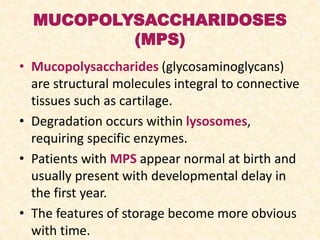
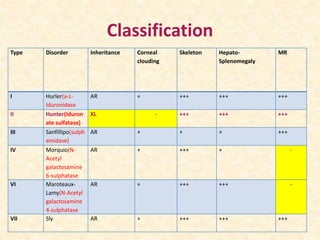
Polysaccharides are polymers of monosaccharides or their derivatives joined by glycosidic bonds. They are classified as homopolymers or heteropolymers. Starch is a major homopolysaccharide composed of amylose and amylopectin, while glycogen is the animal equivalent found mainly in liver and muscle. Cellulose is an insoluble polymer of glucose that provides structure to plant cell walls. Mucopolysaccharides are heteropolymers containing amino sugars and acidic sugars. Common types include hyaluronic acid, chondroitin sulfate, keratin sulfate, heparin, and heparan sulfate. Mucopolysaccharidoses are a group of genetic disorders caused by