Fatty acids ppt - nomenclature & properties- By Sumati Hajela
- 1. FATTY ACIDS – INTRODUCTION, & PROPERTIES
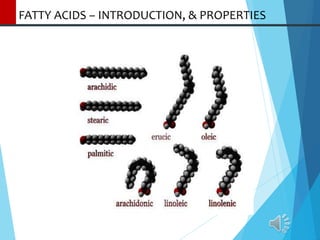
- 2. FATTY ACIDS – INTRODUCTION Fatty acids which occur in natural fats are usually monocarboxylic and contain an even number of C atoms as these are synthesized from two carbon units .These range from 4 to 30 carbon atoms and are straight chain derivatives. The chain may be saturated (containing no double bonds) or unsaturated (containing one or more double bonds) . Some fatty acids may have hydroxyl group(s) in the chain ( hydroxy fatty acids) and still others may possess ring structure (cyclic fatty acids). They are generally present in the esterified form as glycerides and phospholipids. If free, a carboxyl group of a fatty acid will be ionized. Fatty acids differ from each other in a) length of hydrocarbon tail, b) degree of unsaturation, c) position of of double bonds in the chain. Short-chain fatty acids like acetic , propionic and butyric acids are intermediates in metabolism.
- 3. FATTY ACIDS - FUNCTIONS Functions of Fatty Acids: 1) Source of energy in stored forms 2) Solubilize vitamins like A, D, E, and K. 3) Building units of majority of lipids 4) Constituent of phospholipids
- 4. FATTY ACIDS - NOMENCLATURE b)The omega-reference system indicates the number of carbons , the number of double bonds and the position of the double bond closest to the omega carbon, counting from the omega carbon (which is numbered 1 for this purpose). This system is useful in physiological considerations because of the important physiological differences between omega - 3 and omega - 6 fatty acids, and the impossibility to interconvert them in the human body.
- 5. FATTY ACIDS - NOMENCLATURE Greek Letters are Used in Two Ways
- 6. FATTY ACIDS - NOMENCLATURE Summary of the Four Common Systems Here are the four commonly used ways of designating fatty acids.
- 7. FATTY ACIDS - NOMENCLATURE 5) Δx (or delta-x) nomenclature- •Each double bond is indicated by Δx, where the double bond is located on the xth carbon–carbon bond, counting from the carboxylic acid end. • Each double bond is preceded by a cis- or trans- prefix, indicating the conformation of the molecule around the bond. For example, linoleic acid is designated "cis-Δ9, cis-Δ12 octadecadienoic acid". •This nomenclature has the advantage of being less verbose than systematic nomenclature, but is no more technically clear or descriptive.
- 8. FATTY ACIDS - NOMENCLATYRE 6) Lipid numbers- take the form C:D, where C is the number of carbon atoms in the fatty acid and D is the number of double bonds in the fatty acid (if more than one, the double bonds are assumed to be interrupted by CH2 units, i.e., at intervals of 3 carbon atoms along the chain). •This notation can be ambiguous, as some different fatty acids can have the same numbers. Consequently, when ambiguity exists this notation is usually paired with either a Δx or n−x term.
- 9. FATTY ACIDS-CLASSIFICATION Classification of fatty acids:- 1) Saturated Fatty Acid: Contain no double bond, chain is saturated. The molecular formula of a saturated fatty acid is CnH2n+1COOH e.g. palmitic acid is C15H31COOH. e.g. Butyric acid (4), Caproic acid (6) Caprylic acid (8) Capric acid (10), Laurie acid (12), Myristic acid (14), Palmitic acid, (16) Stearic acid (18), Arachidic acid (20) Behenic acid (22) Lignoceric acid (24), Cerotic acid (26) Montanic acid (28) [Figures in the bracket is number of carbons].
- 10. CLASSIFICATION OF SATURATED FATTY ACIDS Common Name Systematic Name Structural Formula Lipid Numbers Propionic acid Propanoic acid CH3CH2COOH C3:0 Butyric acid Butanoic acid CH3(CH2)2COOH C4:0 Valeric acid Pentanoic acid CH3(CH2)3COOH C5:0 Caproic acid Hexanoic acid CH3(CH2)4COOH C6:0 Caprylic acid Octanoic acid CH3(CH2)6COOH C8:0 Capric acid Decanoic acid CH3(CH2)8COOH C10:0 Lauric acid Dodecanoic acid CH3(CH2)10COOH C12:0 Myristic acid Tetradecanoic acid CH3(CH2)12COOH C14:0 Palmitic acid Hexadecanoic acid CH3(CH2)14COOH C16:0 Stearic acid Octadecanoic acid CH3(CH2)16COOH C18:0 Arachidic acid Eicosanoic acid CH3(CH2)18COOH C20:0
- 11. CLASSIFICATION OF UNSATURATED FATTY ACIDS 2) Unsaturated Fatty Acids: Contain one to six double bonds in chain and degree of unsaturation depends on the no. of double bonds present in it. These are sub classified on the basis of degree of unsaturation. •Monoethenoid Acids [ monounsaturated fatty acids] (MUFAs): Contain one double bond e.g. oleic acid, palmitoleic acid . •Polyunsaturated fatty acids(PUFAs)- The double bonds alternate with –CH2 groups (nonconjugated double- bond system) in polyenoic fatty acids and are usually of the ‘cis’ type. These are divided as follows- a) Diethenoid Acids: Contain two double bonds e.g. Linoleic acid. b) Triethenoid Acids: Contain three double bonds e.g. Linolenic acid, Eleostearic acid c) Tetraethenoid Acids: Contain four double bonds e.g. Arachidonic acid.
- 12. CLASSIFICATION OF UNSATURATED FATTY ACIDS ω−n Common Name Lipid Numbers Δn Structural Formula Trans or Cis Naturally Occurring in ω−3 α-Linolenic acid C18:3 Δ9,12,15 CH3CH2CH= CHCH2CH= CHCH2CH= CH(CH2)7C OOH cis Flaxseeds, chiaseeds, walnuts ω−3 Eicosapent aenoic acid C20:5 Δ5,8,11,14,17 CH3CH2CH= CHCH2CH= CHCH2CH= CHCH2CH= CHCH2CH= CH(CH2)3C OOH cis cod liver, herring, mackerel, salmon, menhaden and sardine ω−6 Linoleic acid C18:2 Δ9,12 CH3(CH2)4C H=CHCH2C H=CH(CH2) 7COOH cis Peanut oil, chicken fat, olive oil
- 13. CLASSIFICATION OF FATTY ACIDS Essential Fatty Acids: Fatty acids which cannot be synthesized by the cells of the body must be obtained from other source. Mammals lack the enzymes to introduce double bonds at carbon atoms beyond C9.Hence, all fatty acids containing a double bond at positions beyond C9 have to be supplied in the diet. These are called Essential fatty acids (EFA) . Important essential fatty acids are •Linoleic(18:2 ∆ 9,12) •Linolenic (18:3 ∆ 9,12,15) •Arachidonic acid (20:4 ∆ 5,8,11,14)
- 14. PROPERTIES OF FATTY ACIDS- The length and degree of unsaturation of the hydrocarbon chain determines many of the properties of the fatty acids. Properties may be studied under following headings :- 1.SOLUBILITY IN WATER-Solubility in water decreases with increase in chain length and degree of unsaturation of the fatty acids. Short chains are soluble in water owing to the polar nature of the – COOH group , which ionizes at neutral lpH. Higher fatty acids are insoluble in water but soluble in organic solvents. Except acetic acid , all other fatty acids are lighter than water.
- 15. PROPERTIES OF FATTY ACIDS-
- 16. PROPERTIES OF FATTY ACIDS- 2. MELTING (BOILING)POINT- Melting and boiling points increase with increasing chain length but decrease drastically with increase in the number of double bonds. Fatty acids of chain length <8C are liquid at room temperature(R.T.).Saturated fatty acids12:0 to 24:0 have a waxy consistency at R.T. while unsaturated fatty acids of the same chain length are oily liquids . Fats containing fatty acids 18:2 are liquid below 0oC.Saturated chains have an extended, zig- zag configuration at low temperatures, but shorten at higher temperatures owing to rotation of the -C-C- bonds. This explains why biomembranes get thinner with increasing temperatures.
- 17. PROPERTIES OF FATTY ACIDS- 3. ISOMERISM- The presence of double bonds in the fatty acid chain results in the existence of isomers. Cis isomers are common in nature but unstable, while trans isomers are less common but more stable. Most fatty acids in our body are of the cis type. Trans fatty acids are common in the triglycerides of dairy and meat products, and in partially hydrogenated oils. Saturated fatty acids like stearic acid, and unsaturated fatty acids with trans bonds, have linear shapes but the presence of a cis double bond bends the shape of the molecule.
- 18. PROPERTIES OF FATTY ACIDS- AMPHIPATHIC NATURE- The carboxylic ends of fatty acid molecules are hydrophilic and react with the cellular environment, while the ‘R’ chains are hydrophobic and interact with each other. This amphipathic nature is important in the formation and function of micelles and membranes. OXIDATION.- Oxidation of the double bonds in unsaturated fatty acids yield hydroxy, aldehydic and ketonic derivatives that undergo polymerization into resins. While this is a cause of rancidity in fats, oxidation of oils like linseed oil, produces water proof films.
- 19. PROPERTIES OF FATTY ACIDS- HALOGENATION – Halogenation of the double bonds is useful analytically . e.g. the Iodine Number is the number of gms of Iodine required to saturate 100 gms of a fat. Absorption of UV light by the double bonds is a basis for characterizing fats. FORMATION OF ESTERS WITH ALCOHOLS – The esters of fatty acids with the trihydric alcohol , glycerol , are called neutral fats or triglycerides . Esters with some higher monohydroxy alcohols are called waxes.
- 20. PROPERTIES OF FATTY ACIDS- FORMATION OF SOAPS WITH ALKALIES – Fatty acids make soap with alkali. Sodium soaps are hard. Potassium soaps are soft but costly . To make sodium soaps usable as toilet soaps, sodium carbonate or silicate is added in small amounts .This makes soaps lather with even hard water. Soaps used for shaving are usually potassium soaps using coconut oil or palm oil as the source of fatty acids. To make a soap less alkaline and more smooth to the skin, excess of fatty acids are added. Zinc stearate is a soft powder which is non- irritant to the skin and water repellent. It is commonly used in dusting powders . Calcium and magnesium soaps are insoluble in water and do not lather . hard water which contains salts of calcium and magnesium is hence unsuitable for washing purposes.
- 21. PROPERTIES OF FATTY ACIDS- 9-HYDROLYTIC RANCIDITY- On storage fats become rancid. This is caused by the growth of lipase secreting microbes. Lipase splits fats into glycerol and free fatty acids. Fatty acids impart unpleasant odour and flavor to the fat. Fats should not be mixed with water and kept in cold to avoid rancidity. 10-OXIDATIVE RANCIDITY- Oils containing highly unsaturated fatty acids are spontaneously oxidized by atmospheric oxygen at ordinary temperatures. The autoxidation takes place slowly and forms short chain fatty acids(C4-C10) and aldehydes which give rancid taste and odour to the fats. This is observed more frequently in animal fats than in vegetable fats. Vegetable fats contain natural antioxidants which check autoxidation.
- 22. PROPERTIES OF FATTY ACIDS- 11- HYDROGENATION- Unsaturated fatty acids, either free or combined in lipids, react with gaseous hydrogen to yield the saturated fatty acids. The reaction is of great commercial importance since it permits transformation of inexpensive and unsaturated liquid vegetable fats into solid fats. The latter are used in the manufacture of candles, vegetable shortenings and of oleomargarine. Pt, Pd or Ni are used as catalyst in the above reaction.
- 23. PROPERTIES OF FATTY ACIDS- Acrolein test- Fats when heated in the presence of a dehydrating agent NaHSO4 produce an unsaturated aldehyde called Acrolein from the glycerol moiety. Acrolein is easily recognized by its pungent odour and thus forms the basis of the test for the presence of glycerol in Fat molecule.
- 24. CHARACTERISATION OF FATS- Fats are characterized (purity check) or otherwise assessed by determining certain chemical constants for individual fats- • Saponification number – Number of mgs of KOH required to neutralize the free and combined fatty acids in a gm of a given fat is its saponification number. A high saponification number indicates that the fat is made up of low molecular weight fatty acids and vice versa. • Iodine Number- is the number of gms of Iodine required to saturate 100 gms of a fat. Since Iodine is taken up by the double bonds, a high iodine number indicates a high degree of unsaturation of the fatty acids of the fat.
- 25. CHARACTERISATION OF FATS- •Acid number – Number of mgs of KOH required to neutralize the free fatty acids in a gm of fat is known as acid number . The acid number indicates the degree of rancidity of the given fat. •Reichert – Meissl (R. M.) number – The number of mls of 0.1 N alkali required to neutralize the volatile fatty acids ( separated by saponification , acidification and steam distillation of the fat ) contained in 5 gms of the fat is the R.M. number
- 26. CHARACTERISATION OF FATS- Polenske number-It is the number of milliliters of 0.1N KOH required to neutralize the insoluble fatty acids(those which are not volatile with steam distillation) obtained from 5 gm. of fat. Acetyl number-It is the number of milligrams of KOH required to neutralize the acetic acid obtained by saponification of 1 gm of fat after it has been acetylated.(the treatment of fat or fatty acid mixture with acetic anhydride results in acetylation of all alcoholic OH groups.) The acetyl number is thus a measure of the number of OH groups in the fat. For eg. the caster oil has high acetyl number (146)because it has high content of a hydroxy acid, ricinoleic acid.
- 27. • http://en.wikipedia.org/wiki/Fatty_acid References:- • http://library.med.utah.edu/NetBiochem/FattyAcids/4_1a.h tml • en.wikipedia.org/wiki/Fattyacid • http://en.wikipedia.org/wiki/List_of_saturated_fatty_acids • https://www.rpi.eddeu/pt/bcbp/molbiochem/MBWeb/ mb2/part1/fasynthesis.htm#malcoa • Bioc 460 Spring 2008 - Lecture 36 (Miesfeld) • http://www.powershow.com/view/3d84e5- ZmVkM/LIPID_BIOSYNTHESIS_powerpoint_ppt_presentation • www.learningace.com/doc/4760431/.../lect-22-fasynthesis • http://en.wikipedia.org/wiki/Fatty_acid_synthesis
Editor's Notes
- Fatty acids which occur in natural fats are usually monocarboxylic and contain an even number of C atoms as these are synthesized from two carbon units .These range from 4 to 30 carbon atoms and are straight chain derivatives. The chain may be saturated (containing no double bonds) or unsaturated (containing one or more double bonds) . Some fatty acids may have hydroxyl group(s) in the chain ( hydroxy fatty acids) and still others may possess ring structure (cyclic fatty acids). They are generally present in the esterified form as glycerides and phospholipids. If free,a carboxyl group of a fatty acid will be ionized. Fatty acids differ from each other in a) length of hydrocarbon tail, b) degree of unsaturation, c) position of of double bonds in the chain. Short-chain fatty acids like acetic , propionic and butyric acids are intermediates in metabolism.
- Functions of Fatty Acids: 1) Source of energy in stored forms 2) Solubilize vitamins like A, D, E, and K. 3) Building units of majority of lipids 4) Constituent of phospholipids
- The omega-reference system indicates the number of carbons, the number of double bonds and the position of the double bond closest to the omega carbon, counting from the omega carbon (which is numbered 1 for this purpose). This system is useful in physiological considerations because of the important physiological differences between omega - 3 and omega - 6 fatty acids, and the impossibility to interconvert them in the human body.
- Greek Letters are Used in Two Ways Greek letters are used to denote positions relative to the carboxyl carbon. The diagram illustrates how Greek letters are used to denote positions relative to either end of a fatty acid chain. The first carbon following the carboxyl carbon is the alpha carbon. The second carbon following the carboxyl carbon is the beta carbon. The last carbon in the chain, farthest from the carboxyl group, is the omega carbon. Sometimes carbon atoms close to the ω carbon are designated in relation to it E.g., the third from the end is ω-3(omega minus 3 ).
- Summary of the Four Common Systems Here are the four commonly used ways of designating fatty acids.
- 5) Δx (or delta-x) nomenclature,- Each double bond is indicated by Δx, where the double bond is located on the xth carbon–carbon bond, counting from the carboxylic acid end. Each double bond is preceded by a cis- or trans- prefix, indicating the conformation of the molecule around the bond. For example, linoleic acid is designated "cis-Δ9, cis-Δ12 octadecadienoic acid". This nomenclature has the advantage of being less verbose than systematic nomenclature, but is no more technically clear or descriptive.
- 6)Lipid numbers- take the form C:D, where C is the number of carbon atoms in the fatty acid and D is the number of double bonds in the fatty acid (if more than one, the double bonds are assumed to be interrupted by CH2 units, i.e., at intervals of 3 carbon atoms along the chain). This notation can be ambiguous, as some different fatty acids can have the same numbers. Consequently, when ambiguity exists this notation is usually paired with either a Δx or n−x term.
- Classification of fatty acids:- 1) Saturated Fatty Acid: Contain no double bond, chain is saturated. The molecular formula of a saturated fatty acid is CnH2n+1COOH e.g. palmitic acid is C15H31COOH. e.g. Butyric acid (4), Caproic acid (6) Caprylic acid (8) Capric acid (10), Laurie acid (12), Myristic acid (14), Palmitic acid, (16) Stearic acid (18), Arachidic acid (20) Behenic acid (22) Lignoceric acid (24), Cerotic acid (26) Montanic acid (28) [Figures in the bracket is number of carbons].
- This list shows common name , systematic name , structural formula and lipid numbers of few saturated fatty acids .
- 2) Unsaturated Fatty Acid: Contain one to six double bonds in chain and degree of unsaturation depends on the no. of double bonds present in it. These are sub classified on the basis of degree of unsaturation. Monoethenoid Acids [ monounsaturated fatty acids (MUFAs): Contain one double bond e.g. oleic acid, palmitoleic acid . Polyunsaturated fatty acids(PUFAs)- The double bonds alternate with –CH2 groups (nonconjugated double- bond system) in polyenoic fatty acids and are usually of the ‘cis’type..These are divided as follows- a) Diethenoid Acids: Contain two double bonds e.g. Linoleic acid. b) Triethenoid Acids: Contain three double bonds e.g. Linolenic acid, Eleostearic acid c) Tetraethenoid Acids: Contain four double bonds e.g. Arachidonic acid.
- Here is the list of some common unsaturated fatty acids.
- Essential Fatty Acids:Fatty acids which cannot be synthesized by the cell of the body must be obtained from other source. Mammals lack the enzymes to introduce double bonds at carbon atoms beyond C9.Hence, all fatty acids containing a double bond at positions beyond C9 have to be supplied in the diet. These are called Essential fatty acids (EFA) . Important essential fatty acids are :- Linoleic Linolenic and Arachidonic acid.
- Properties of fatty acids : SOLUBILITY IN WATER- MELTING (BOILING)POINT- ISOMERISM- AMPHIPATHIC NATURE- OXIDATION.- HALOGENATION 7. FORMATION OF ESTERS WITH ALCOHOLS –8 . F 8-ORMATION OF SOAPS WITH ALKALIES – Fatty acids make soap with alkali.
- Properties of fatty acids : SOLUBILITY IN WATER- MELTING (BOILING)POINT- ISOMERISM- AMPHIPATHIC NATURE- OXIDATION.- HALOGENATION 7. FORMATION OF ESTERS WITH ALCOHOLS –8 . F 8-ORMATION OF SOAPS WITH ALKALIES – Fatty acids make soap with alkali.
- Properties of fatty acids : SOLUBILITY IN WATER- MELTING (BOILING)POINT- ISOMERISM- AMPHIPATHIC NATURE- OXIDATION.- HALOGENATION 7. FORMATION OF ESTERS WITH ALCOHOLS –8 . F 8-ORMATION OF SOAPS WITH ALKALIES – Fatty acids make soap with alkali.
- Properties of fatty acids : SOLUBILITY IN WATER- MELTING (BOILING)POINT- ISOMERISM- AMPHIPATHIC NATURE- OXIDATION.- HALOGENATION 7. FORMATION OF ESTERS WITH ALCOHOLS –8 . F 8-ORMATION OF SOAPS WITH ALKALIES – Fatty acids make soap with alkali.
- Properties of fatty acids : SOLUBILITY IN WATER- MELTING (BOILING)POINT- ISOMERISM- AMPHIPATHIC NATURE- OXIDATION.- HALOGENATION 7. FORMATION OF ESTERS WITH ALCOHOLS –8 . F 8-ORMATION OF SOAPS WITH ALKALIES – Fatty acids make soap with alkali.
- Properties of fatty acids : SOLUBILITY IN WATER- MELTING (BOILING)POINT- ISOMERISM- AMPHIPATHIC NATURE- OXIDATION.- HALOGENATION 7. FORMATION OF ESTERS WITH ALCOHOLS –8 . F 8-ORMATION OF SOAPS WITH ALKALIES – Fatty acids make soap with alkali.
- Properties of fatty acids : SOLUBILITY IN WATER- MELTING (BOILING)POINT- ISOMERISM- AMPHIPATHIC NATURE- OXIDATION.- HALOGENATION 7. FORMATION OF ESTERS WITH ALCOHOLS – 8-FORMATION OF SOAPS WITH ALKALIES – Fatty acids make soap with alkali.
- Properties of fatty acids : SOLUBILITY IN WATER- MELTING (BOILING)POINT- ISOMERISM- AMPHIPATHIC NATURE- OXIDATION.- HALOGENATION 7. FORMATION OF ESTERS WITH ALCOHOLS – 8-FORMATION OF SOAPS WITH ALKALIES – Fatty acids make soap with alkali. 9-HYDROLYTIC RANCIDITY 10-OXIDATIVE RANCIDITY 11-HYDROGENATION 12-ACROLEIN TEST
- Properties of fatty acids : SOLUBILITY IN WATER- MELTING (BOILING)POINT- ISOMERISM- AMPHIPATHIC NATURE- OXIDATION.- HALOGENATION 7. FORMATION OF ESTERS WITH ALCOHOLS – 8-FORMATION OF SOAPS WITH ALKALIES – Fatty acids make soap with alkali. 9-HYDROLYTIC RANCIDITY 10-OXIDATIVE RANCIDITY 11-HYDROGENATION 12-ACROLEIN TEST
- Properties of fatty acids : SOLUBILITY IN WATER- MELTING (BOILING)POINT- ISOMERISM- AMPHIPATHIC NATURE- OXIDATION.- HALOGENATION 7. FORMATION OF ESTERS WITH ALCOHOLS – 8-FORMATION OF SOAPS WITH ALKALIES – Fatty acids make soap with alkali. 9-HYDROLYTIC RANCIDITY 10-OXIDATIVE RANCIDITY 11-HYDROGENATION 12-ACROLEIN TEST
- Characterisation of fats Fats are characterised and their purity or otherwise assessed by determining certain chemical constants for individual fats- Saponification number – Number of mgs of KOH required to neutralize the free and combined fatty acids in a gm of a given fat is its saponification number. A high saponification number indicates that the fat is made up of low molecular weight fatty acids and vice versa. Iodine Number- is the number of gms of Iodine required to saturate 100 gms of a fat. Since Iodine is taken up by the double bonds, a high iodine number indicates a high degree of unsaturation of the fatty acids of the fat.
- Characterisation of fats Acid number – Number of mgs of KOH required to neutralize the free fatty acids in a gm of fat is known as acid number . The acid number indicates the degree of rancidity of the given fat. Reichert – Meissl (R. M.) number – The number of mls of 0.1 N alkali required to neutralise the volatile fatty acids ( separated by saponification , acidification and steam distillation of the fat ) contained in 5 gms of the fat is the R.M. number
- Characterisationof fats Polenske number-It is the number of milliliters of 0.1N KOH required to neutralize the insoluble fatty acids(those which are not volatile with steam distillation) obtained from 5 gm. of fat. Acetyl number-It is the number of milligrams of KOH required to neutralize the acetic acid obtained by saponification of 1 gm of fat after it has been acetylated.(the treatment of fat or fatty acid mixture with acetic anhydride results in acetylation of all alcoholic OH groups.) The acetyl number is thus a measure of the number of OH groups in the fat. For eg. the caster oil has high acetyl number (146)because it has high content of a hydroxy acid, ricinoleic acid.