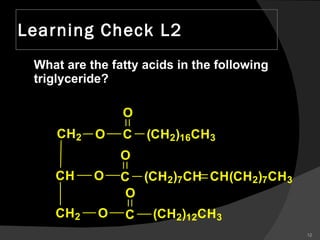
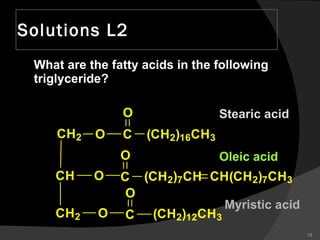
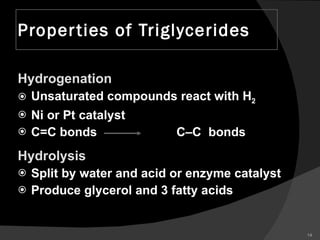
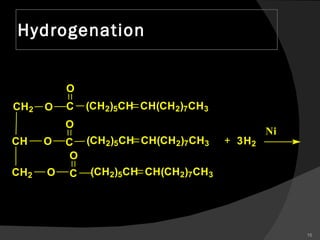
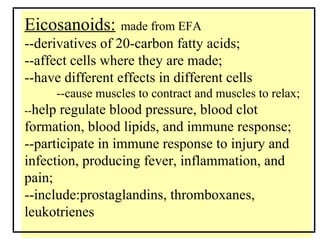
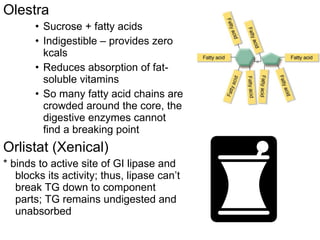
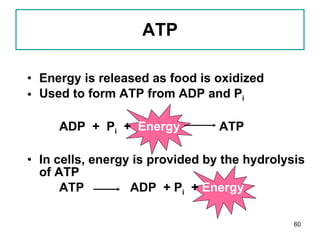
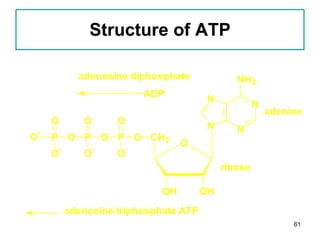
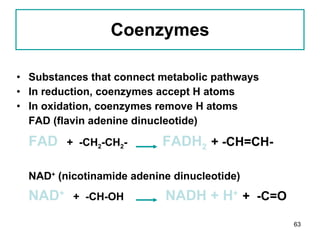
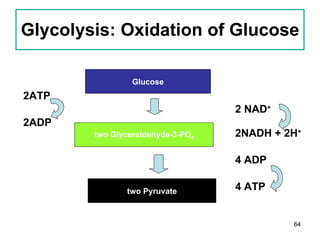
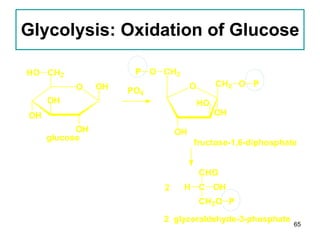
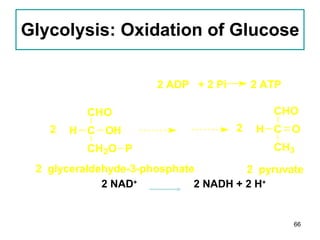
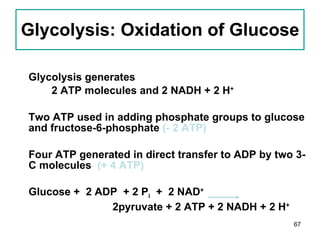
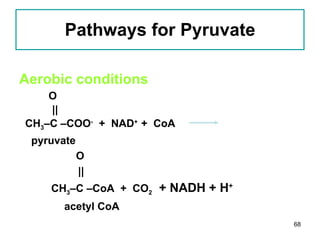
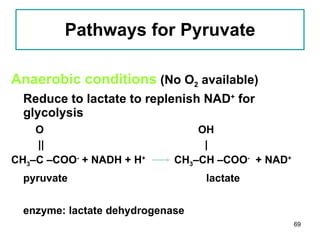
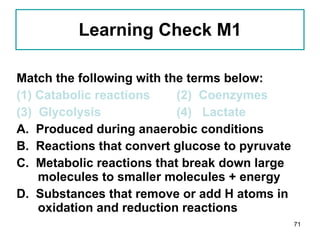
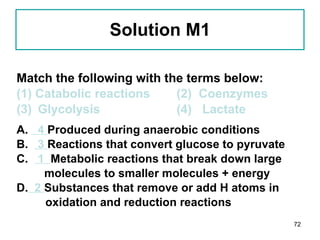
1. The document discusses different types of lipids including fatty acids, triglycerides, phospholipids, and steroids.
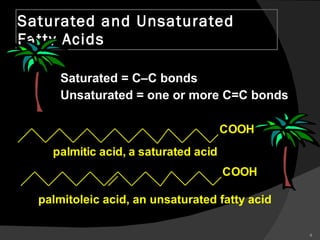
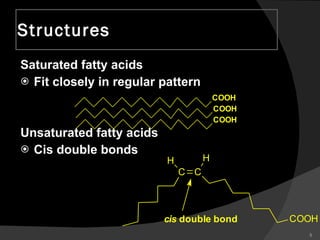
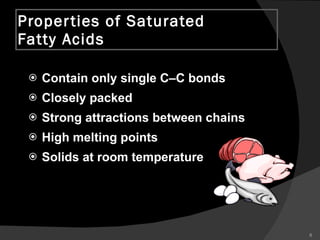
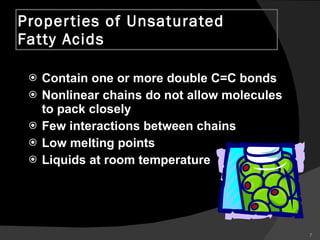
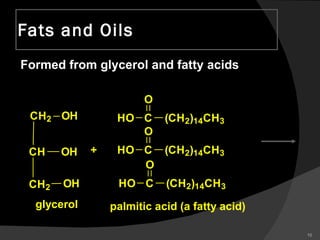
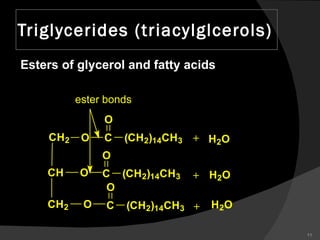
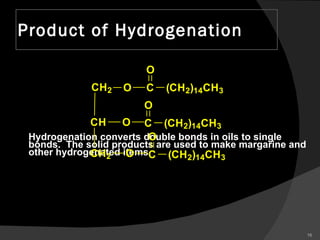
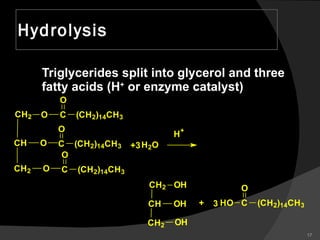
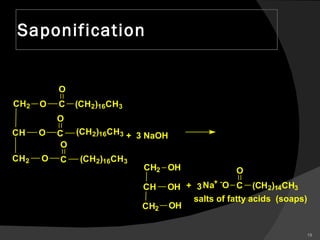
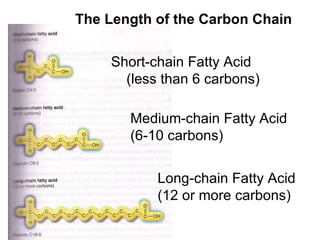
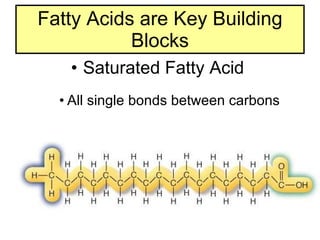
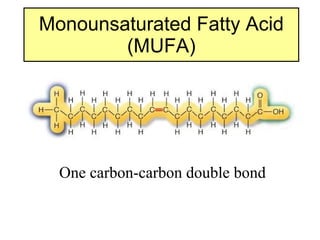
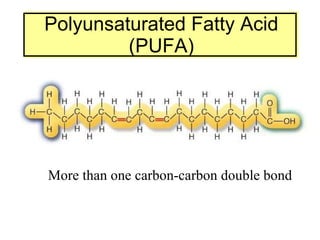
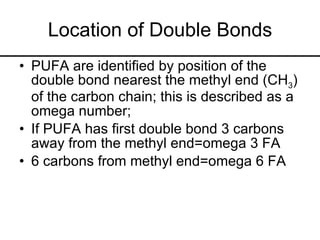
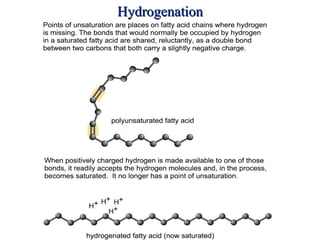
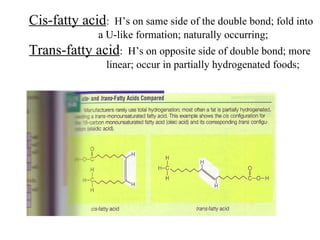
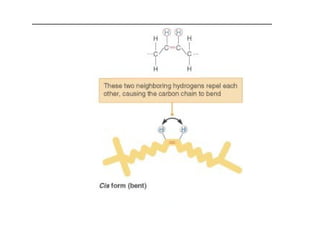
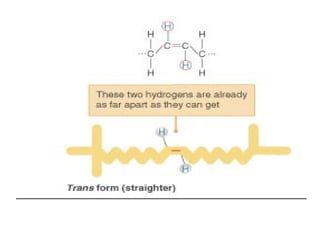
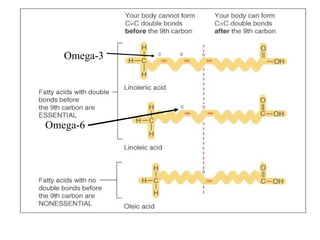
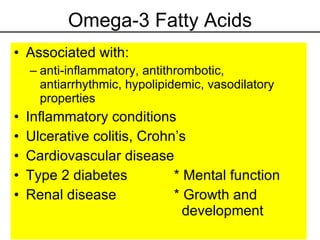
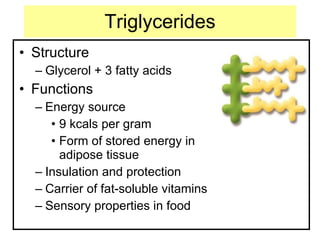
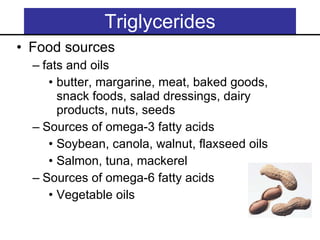
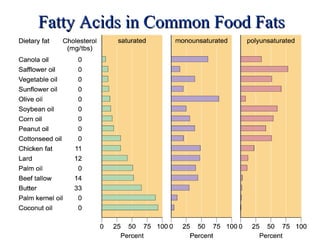
2. It explains the structures and properties of saturated and unsaturated fatty acids. Triglycerides are formed from glycerol and three fatty acids and are a major form of fat storage.
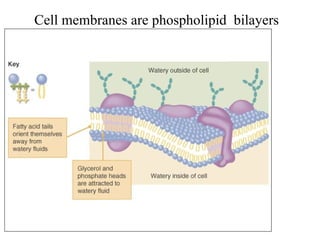
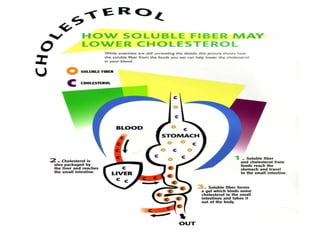
3. Phospholipids are a major component of cell membranes and contain a phosphate group. Cholesterol is an important sterol that is a component of cell membranes and precursor for other substances.