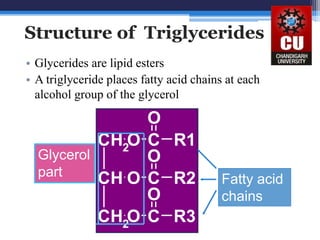
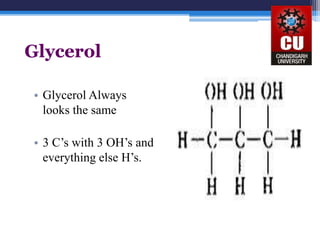
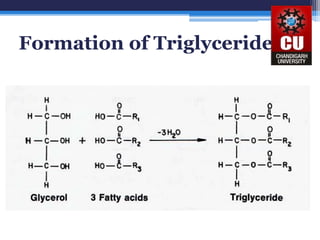
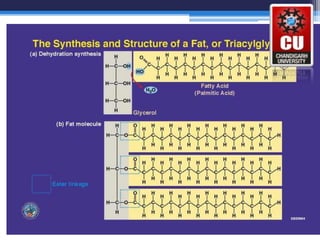
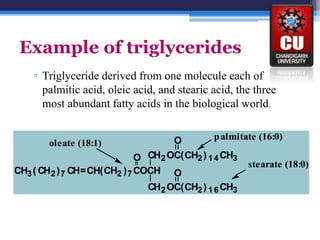
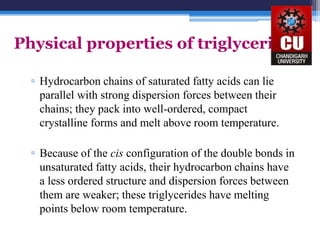
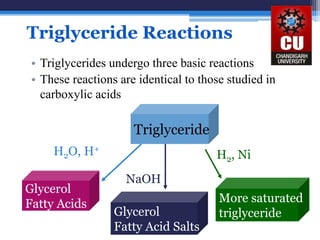
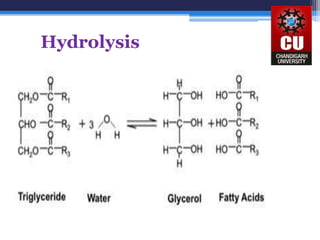
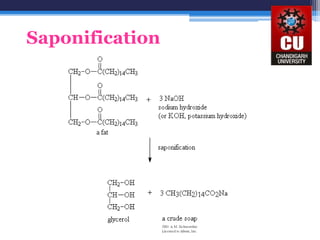
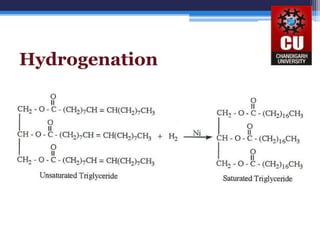
Triglycerides are composed of glycerol bonded to three fatty acid chains. They are the main constituents of body fats and oils. Triglyceride structure consists of a glycerol backbone bonded to fatty acid chains through ester linkages. The physical properties of triglycerides, such as melting point, depend on the length and saturation of the fatty acid chains. Saturated fatty acid chains pack tightly, causing triglycerides with more saturated chains to be solid at room temperature. Unsaturated chains do not pack as tightly, making triglycerides with more unsaturated chains liquid at room temperature. Triglycerides undergo three main reactions: hydrolysis, saponification, and hydrogenation.