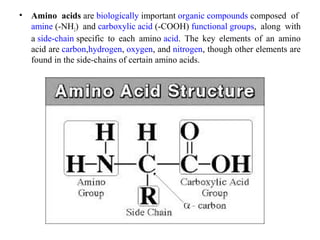
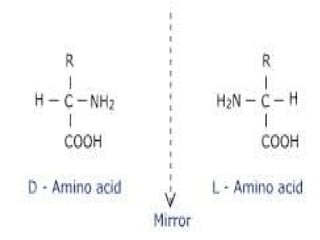
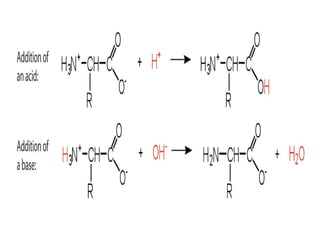
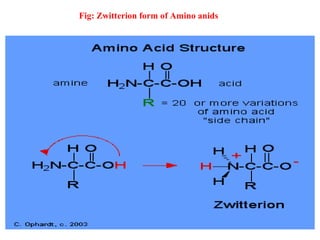
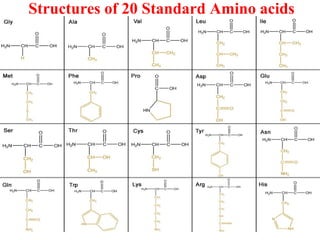
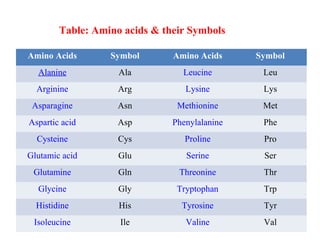
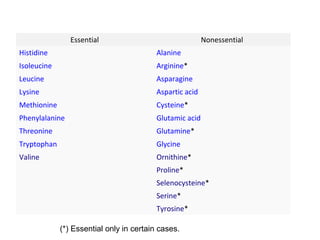
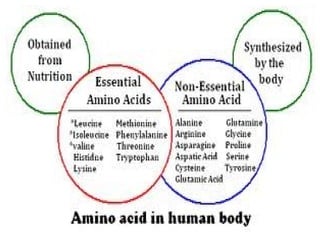
Amino acids are organic compounds with amine and carboxyl groups, serving as the building blocks of proteins and exhibiting amphoteric properties. They can exist in zwitterion form and are classified based on various criteria, including dietary necessity and side-chain composition. The 20 standard amino acids are crucial for protein structure, while many non-standard amino acids also play important biological roles.