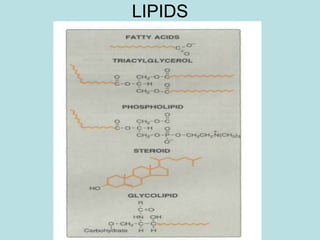
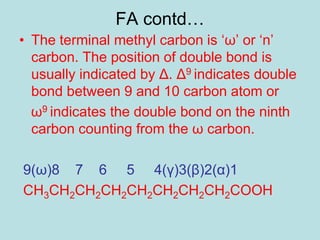
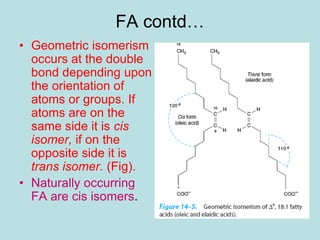
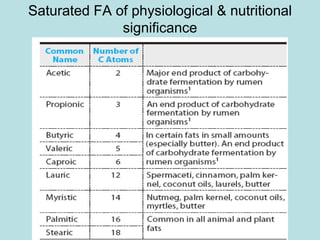
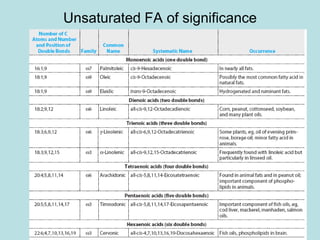
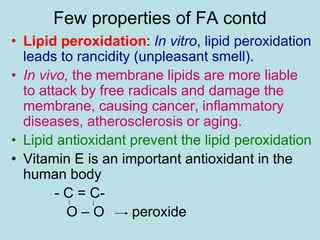
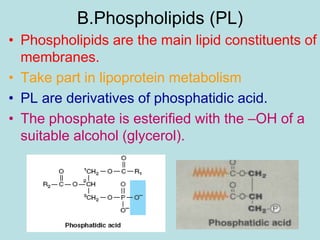
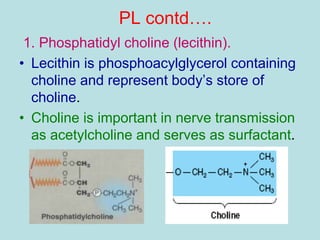
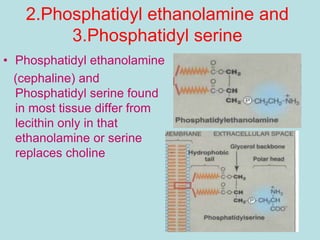
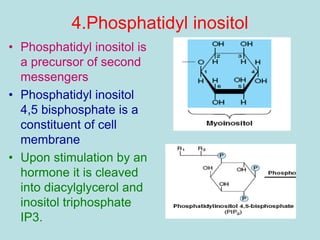
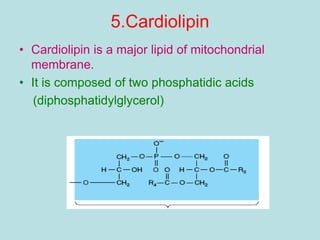
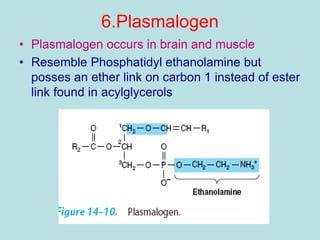
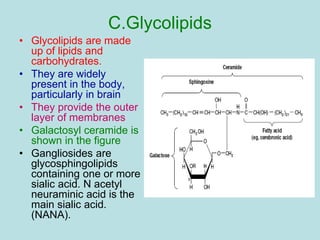
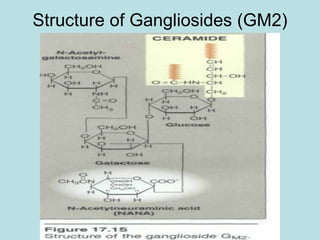
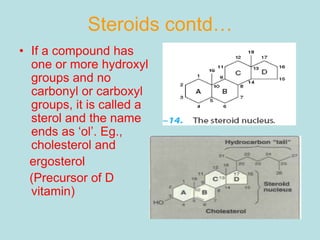
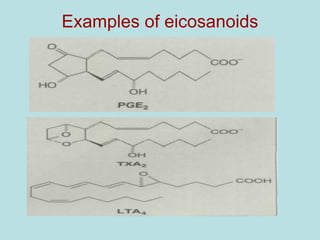
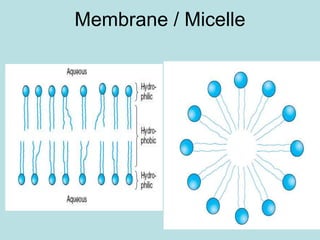
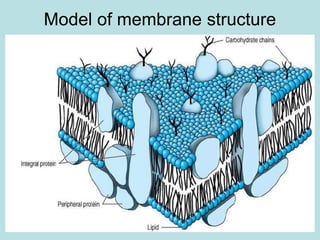
Lipids are a diverse group of hydrophobic organic molecules crucial for energy, structural components of membranes, and various biological functions. They are classified into simple, complex, and derived lipids, with fatty acids, triglycerides, and phospholipids playing significant roles in metabolism and cell structure. Eicosanoids and antioxidants like vitamin E are also important, as they contribute to cellular processes and protect against oxidative damage.