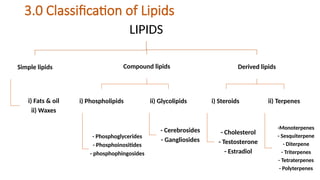
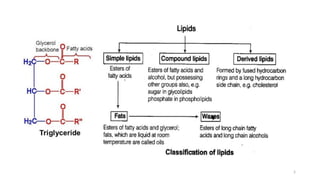
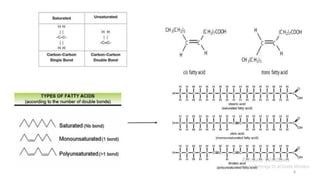
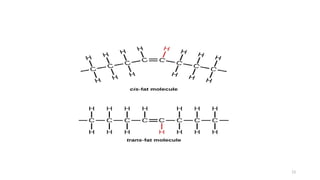
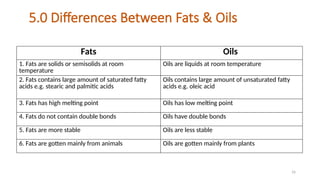
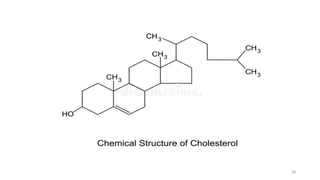
The document provides a comprehensive overview of lipids, detailing their types, functions, and classifications, including fatty acids' characteristics. It explains the roles lipids play in biological systems, their energy storage capacity, and their structural importance in cell membranes. Additionally, the document discusses the differences between fats and oils, as well as their physical and chemical properties.