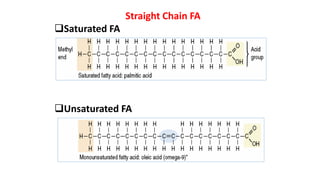
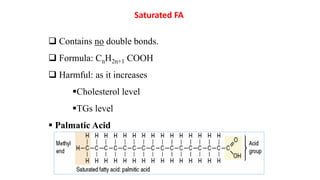
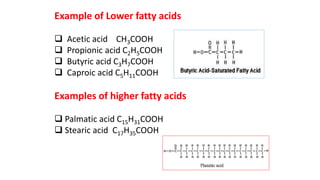
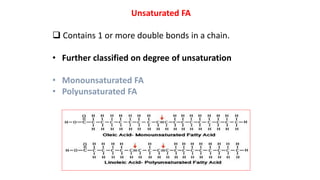
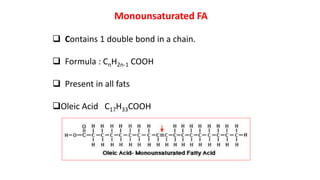
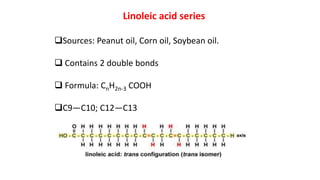
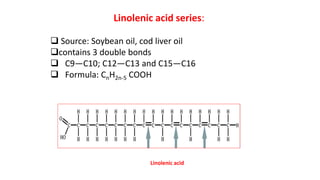
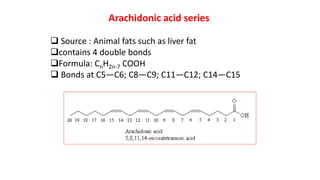
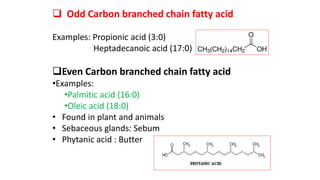
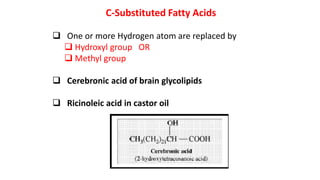
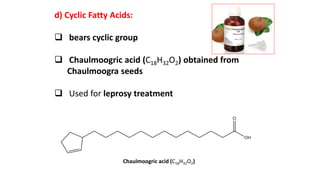
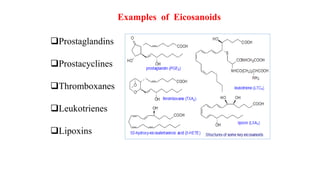
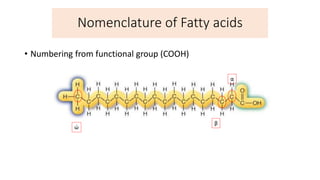
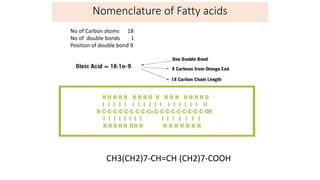
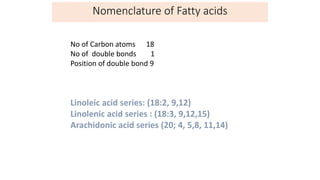
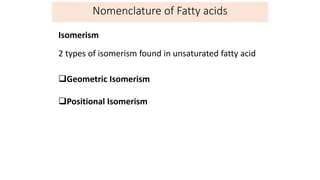
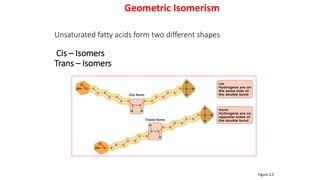
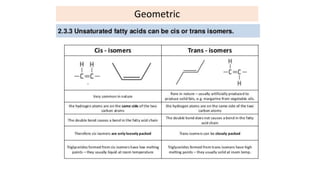
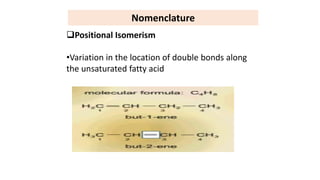
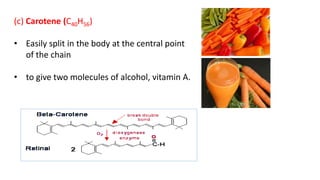
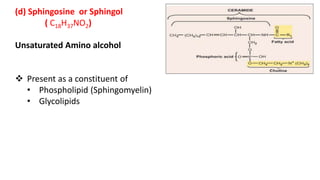
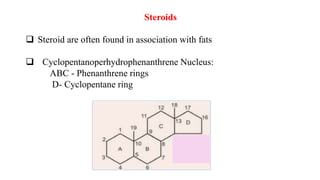
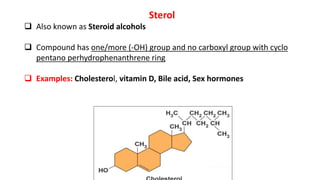
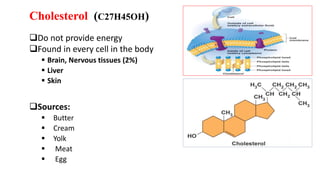
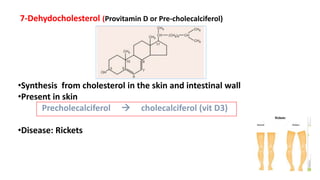
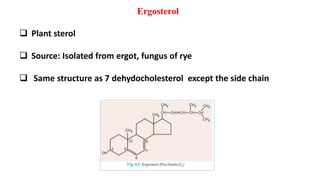
This document defines and provides examples of derived lipids, including fatty acids and glycerol. It discusses the classification, nomenclature, and importance of fatty acids. Specifically, it describes saturated and unsaturated fatty acids, essential fatty acids like linoleic acid, and eicosanoids derived from arachidonic acid. It also discusses glycerol as a derived lipid and important alcohols like phytol, carotene, and sphingosine. Finally, it covers cholesterol as a key steroid and provides examples of other plant and animal sterols.