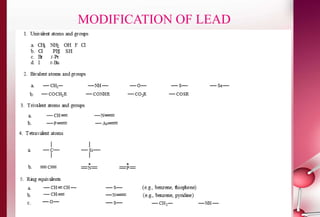
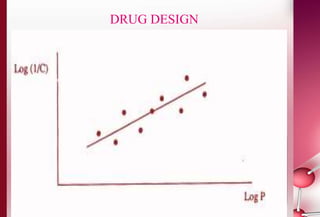
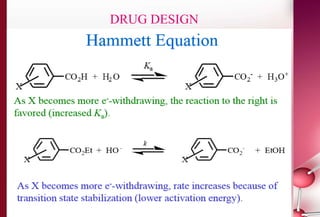
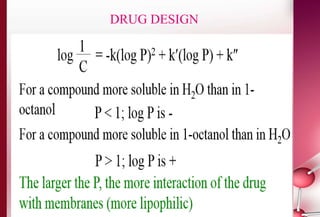
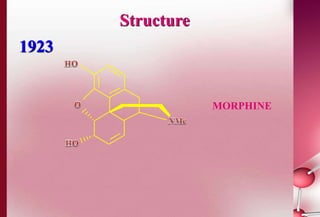
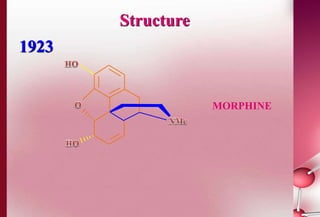
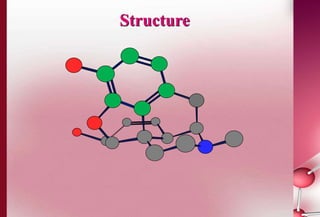
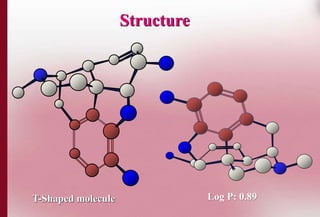
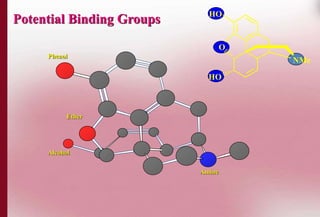
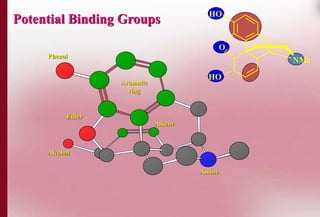
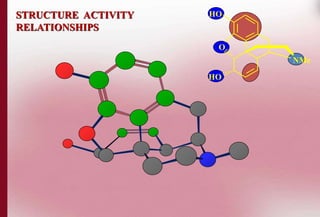
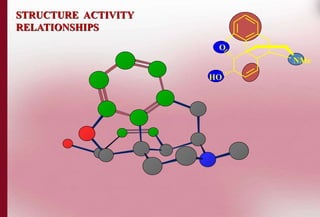
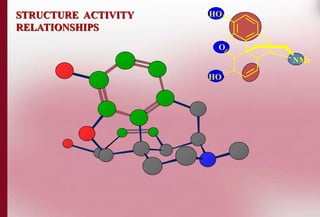
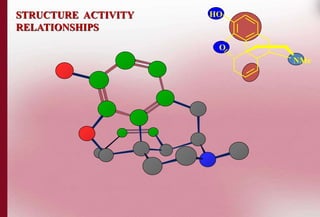
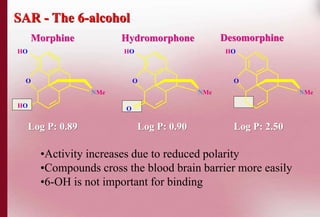
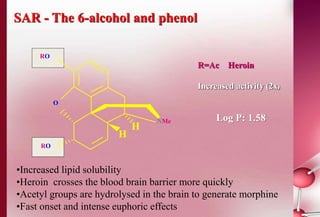
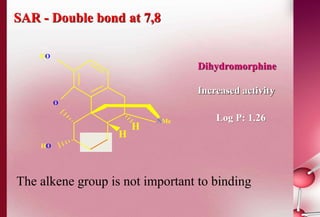
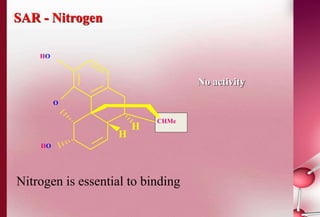
This document discusses structure-activity relationships in drug design and formulation. It introduces Hammett and Hansch plots, which relate reaction rates and biological activity to electronic and physicochemical properties. Modification of lead compounds is explored through changing functional groups, stereochemistry and lipophilicity. Morphine is used as a case study to illustrate how properties like log P, binding groups and stereochemistry impact opioid activity. The conclusion emphasizes the role of medicinal chemistry in understanding disease and developing safer, more effective pharmaceuticals.




















![REFERENCES
• ANON, 2014. Assessment of chemicals. Introduction to (Quantitative)
structure activity relationships [online]. Available from:
http://www.oecd.org/chemicalsafety/risk-assessment/
introductiontoquantitativestructureactivityrelationships.htm
• MCKINNEY, J.D. et al, 2000. Toxicological sciences. The practice of
structure activity relationships (SAR) in toxicology [online], 56(1), 8-17.
Available from: http://toxsci.oxfordjournals.org/content/56/1/8.full
• PARIKH, 2009. Medicinal Chemistry. The SAR & QSAR approaches to
drug design [online]. Available from:
http://faculty.mville.edu/parikhs/courses/chm2004/lecture%20notes/CHM
%202004%20Lectures%20-%20Chapter%204.pdf
• TOROK, B. Medicinal chemistry. SAR and QSAR [online]. Available
from:
http://alpha.chem.umb.edu/chemistry/ch458/files/Lecture_Slides/Lecture_
Chapter_3.pdf [Accessed on 8 November 2014].
• MANIBUSAN, M. et al, 2012. Technical working group on pesticides.
(Quantitative) structure activity relationship [(Q)SAR] guidance
document [online]. Available from:
http://www.epa.gov/oppfead1/international/naftatwg/guidance/qsar-guidance.
pdf](https://image.slidesharecdn.com/sar-morphine-141111042006-conversion-gate01/85/SAR-of-Morphine-53-320.jpg)
![REFERENCES
• ANON, 2014. Wikipedia. Louis Plack Hammett [online]. Available
from: http://en.wikipedia.org/wiki/Louis_Plack_Hammett
• ANON, 2014. Wikipedia. Corwin Hansch [online]. Available from:
http://en.wikipedia.org/wiki/Corwin_Hansch
• Medicinal Chemistry- Chapter 3, QSAR of Morphine. Available at:
http://carbon.indstate.edu/rfitch/CHEM%20452/Chapter_3.pdf
• Anon, Morphine Chemistry, Online. Available at:
http://www.emsb.qc.ca/laurenhill/science/morphine.html
• Anon, 2012, A Look at the Morphinan Structure Activity
Relationships of Six Popular Opiates, Online. Available at:
http://opiophilia.blogspot.com/2012/12/opiate-structure-activity-relationship.
html
• Florencio Zaragoza Dörwald : Lead Optimization for Medicinal
Chemists: Pharmacokinetic Properties of Functional Groups and
Organic Compounds](https://image.slidesharecdn.com/sar-morphine-141111042006-conversion-gate01/85/SAR-of-Morphine-54-320.jpg)
