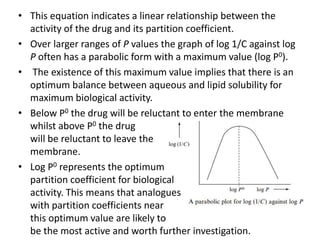
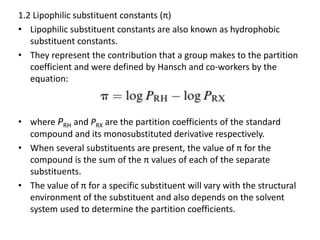
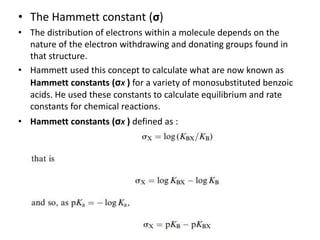
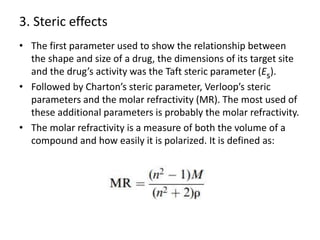
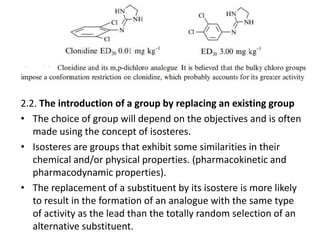
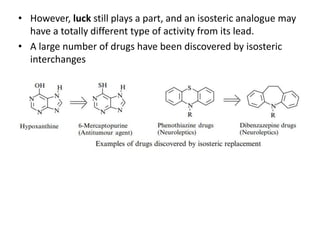
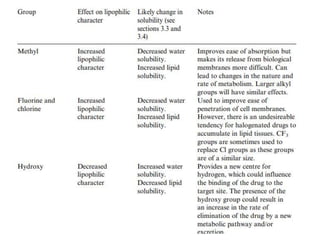
This document discusses structure-activity relationships (SARs) and quantitative structure-activity relationships (QSARs) in drug design. It explains that compounds with similar structures to an active drug are often biologically active as well, and studying their SARs can provide information about what parts of the structure are responsible for the drug's effects and side effects. QSARs attempt to mathematically relate biological activity to measurable physicochemical parameters like lipophilicity, electronic effects, and steric effects. Parameters like partition coefficients, Hammett constants, and Taft steric constants are used to represent these properties in equations. Hansch analysis and Craig plots are also discussed as methods to predict potential drug activity based on these quantitative relationships.




![• The parameters commonly used to represent these properties
are partition coefficients for lipohilicity, Hammett σ
constants for electronic effects and Taft Ms steric constants
for steric effects.
• QSAR derived equations take the general form:
biological activity = function{parameter(s)}
in which the activity is normally expressed as
log[1/(concentration term)], usually C, the minimum
concentration required to cause a defined biological response
1. Liphophilicity
Two parameters are commonly used to represent
lipophilicity:
1.1 Partition coefficient (P)
1.2 Lipophilicity substituent constant (π).
The partition coefficient parameter refers to the whole
molecule while the latter is related to substituent groups.](https://image.slidesharecdn.com/principlesandapplicationsofstructureactivityrelationship-seminar-220421071111/85/Principles-and-Applications-of-Structure-Activity-Relationship-10-320.jpg)