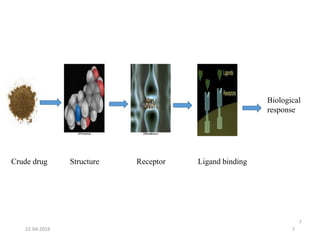
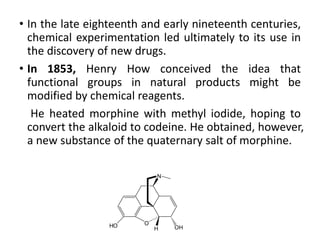
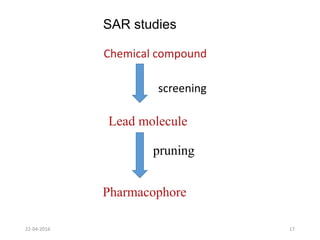
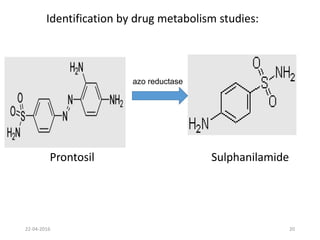
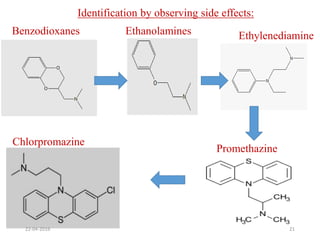
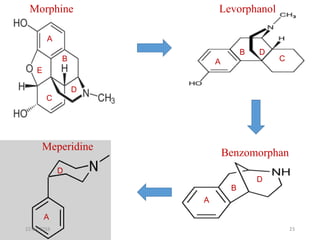
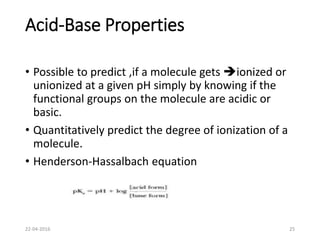
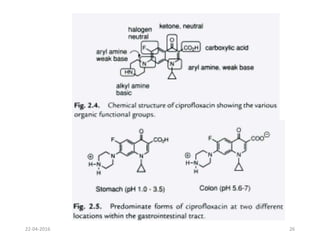
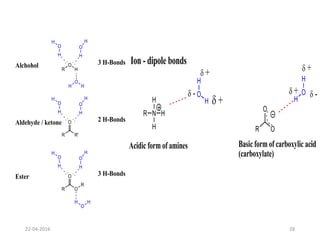
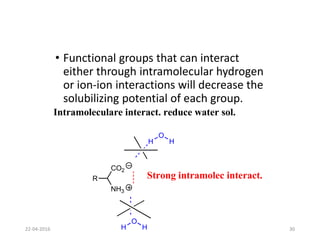
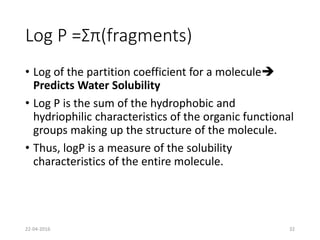
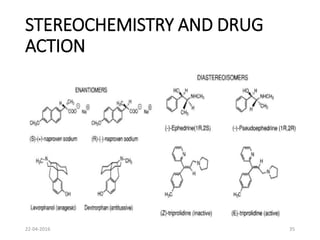
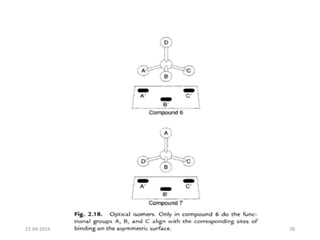
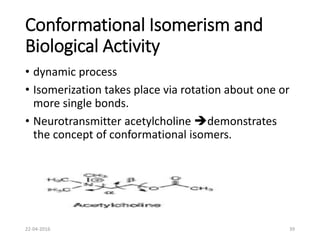
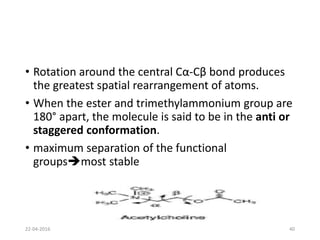
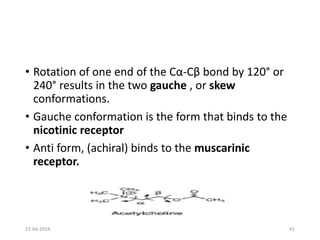
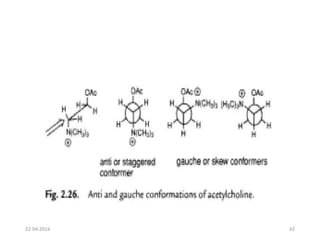
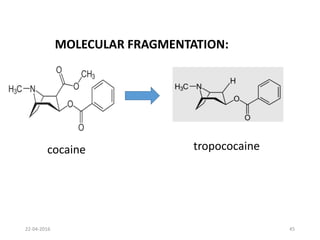
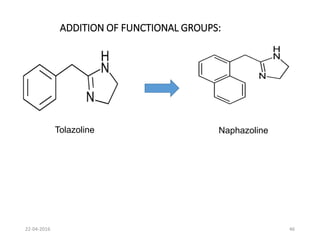
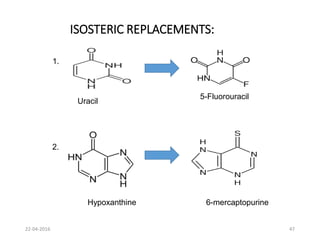
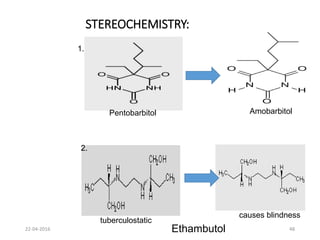
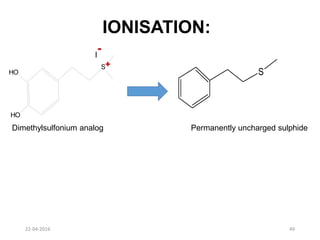
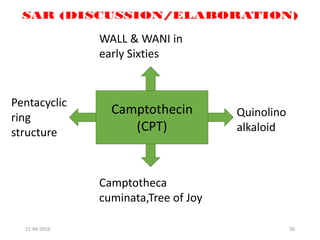
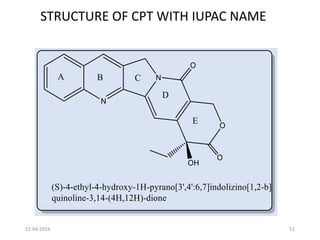
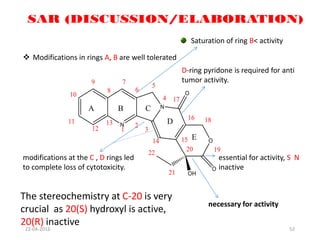
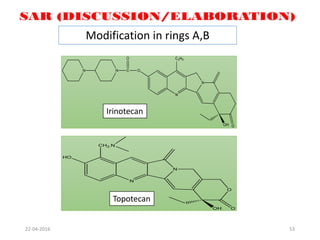
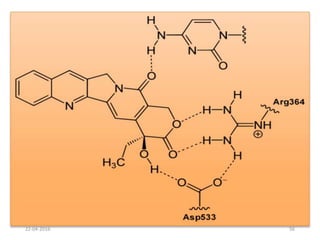
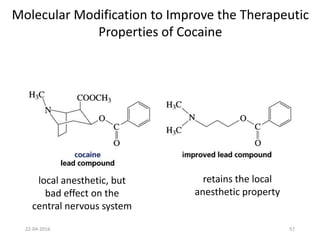
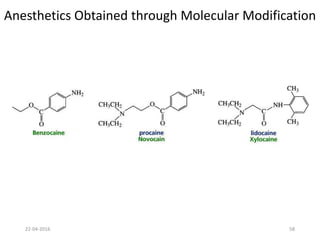
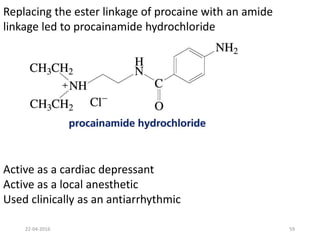
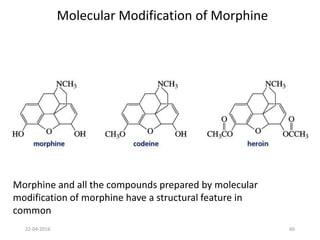
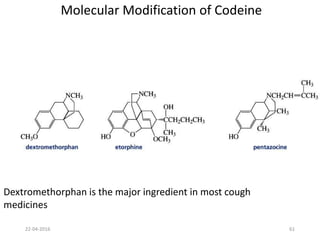
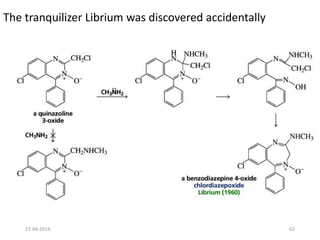
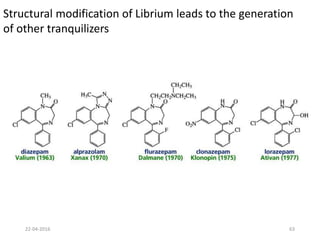
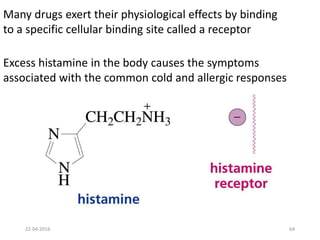
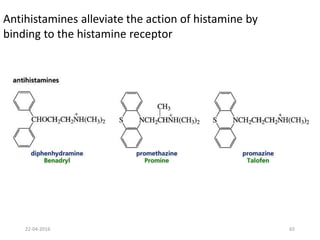
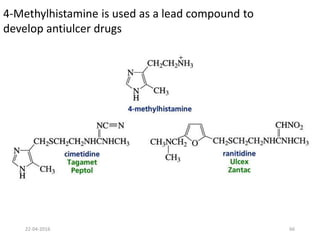
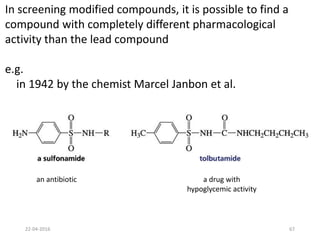
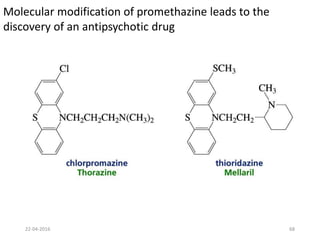
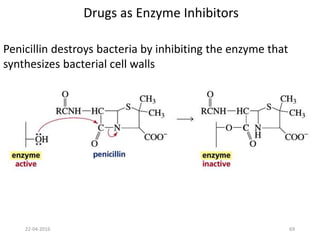
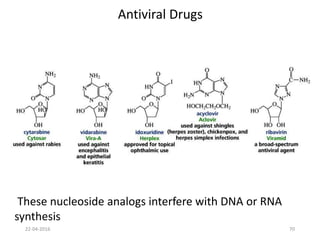
This document discusses structure-activity relationships (SAR) and how medicinal chemistry uses SAR to develop new drugs. It defines SAR as the relationship between a molecule's structure and its biological activity. Medicinal chemists analyze how structural modifications affect biological properties in order to determine which parts of a molecule are responsible for its effects. The goal is to optimize desired effects and minimize unwanted side effects by making targeted changes to a lead compound's structure. The document provides several examples of drugs that were developed through systematic SAR studies of existing compounds.