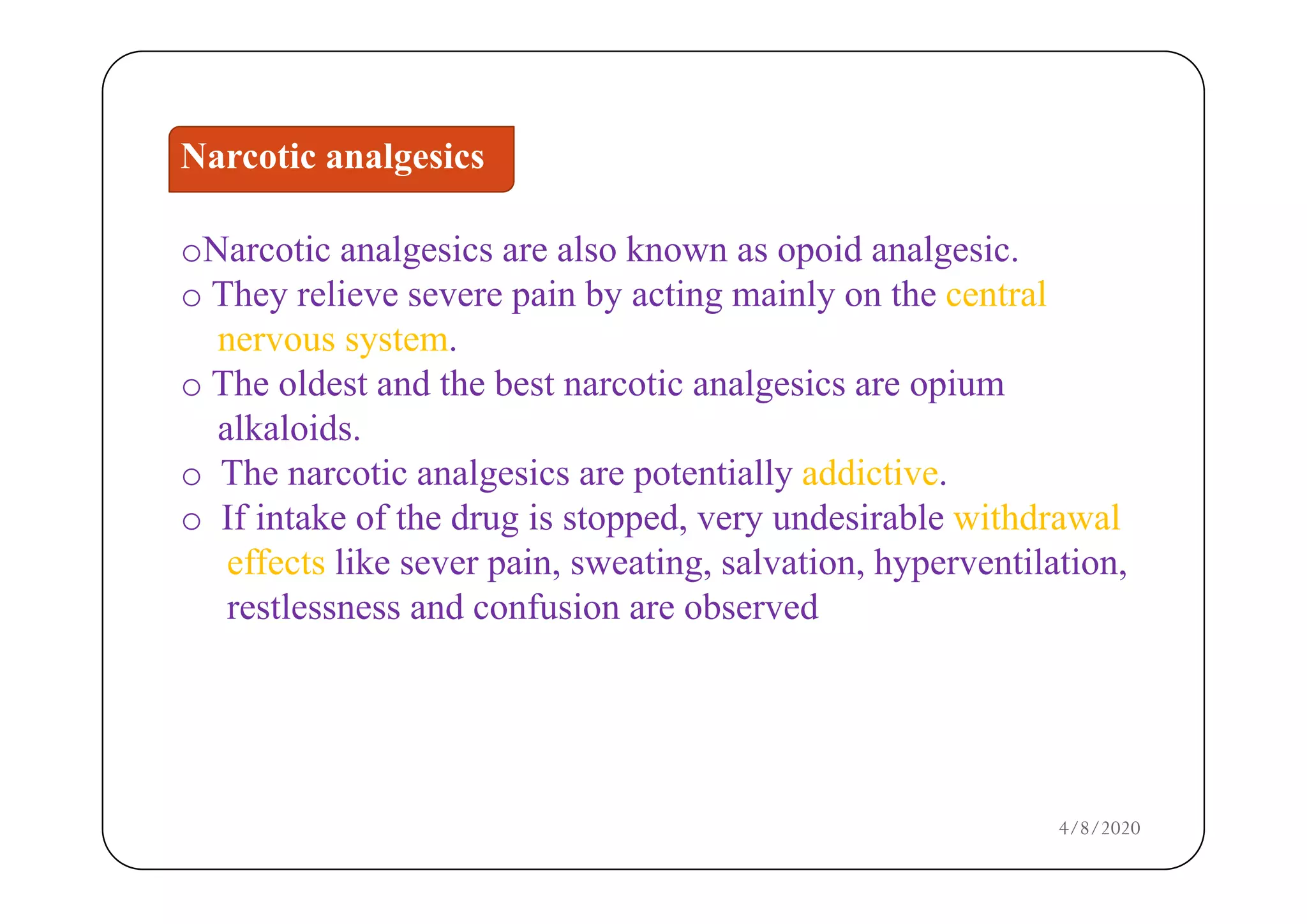
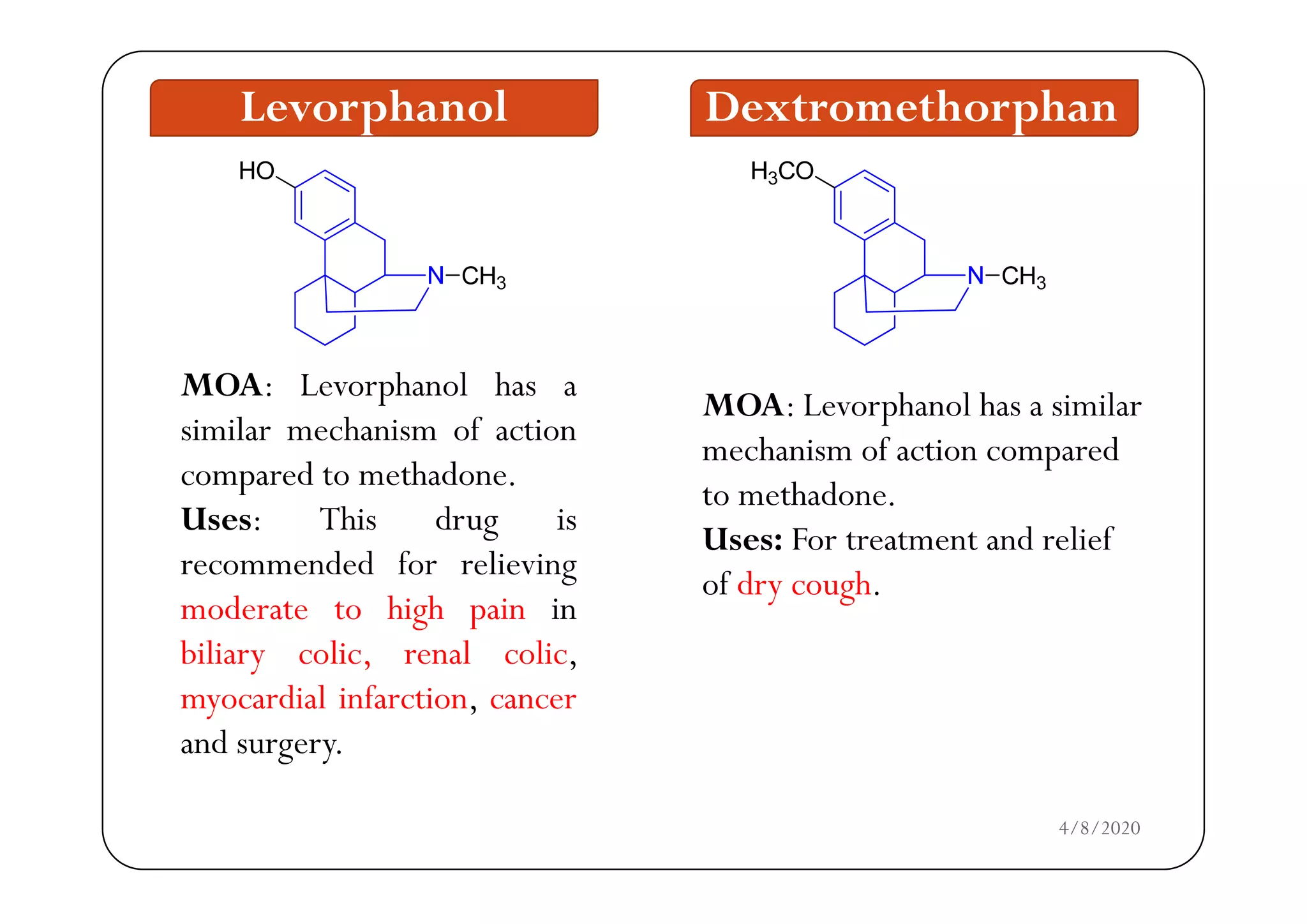
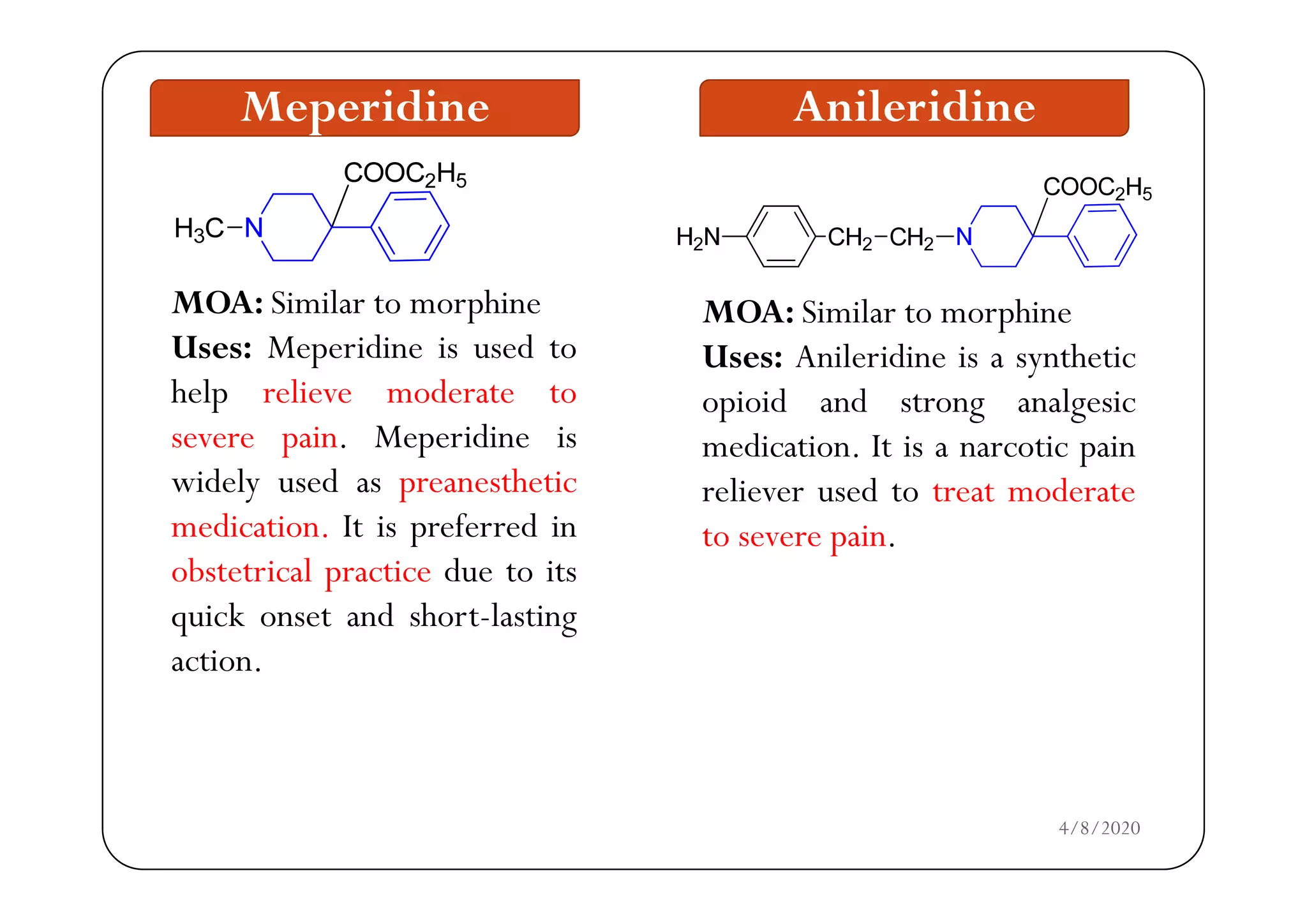
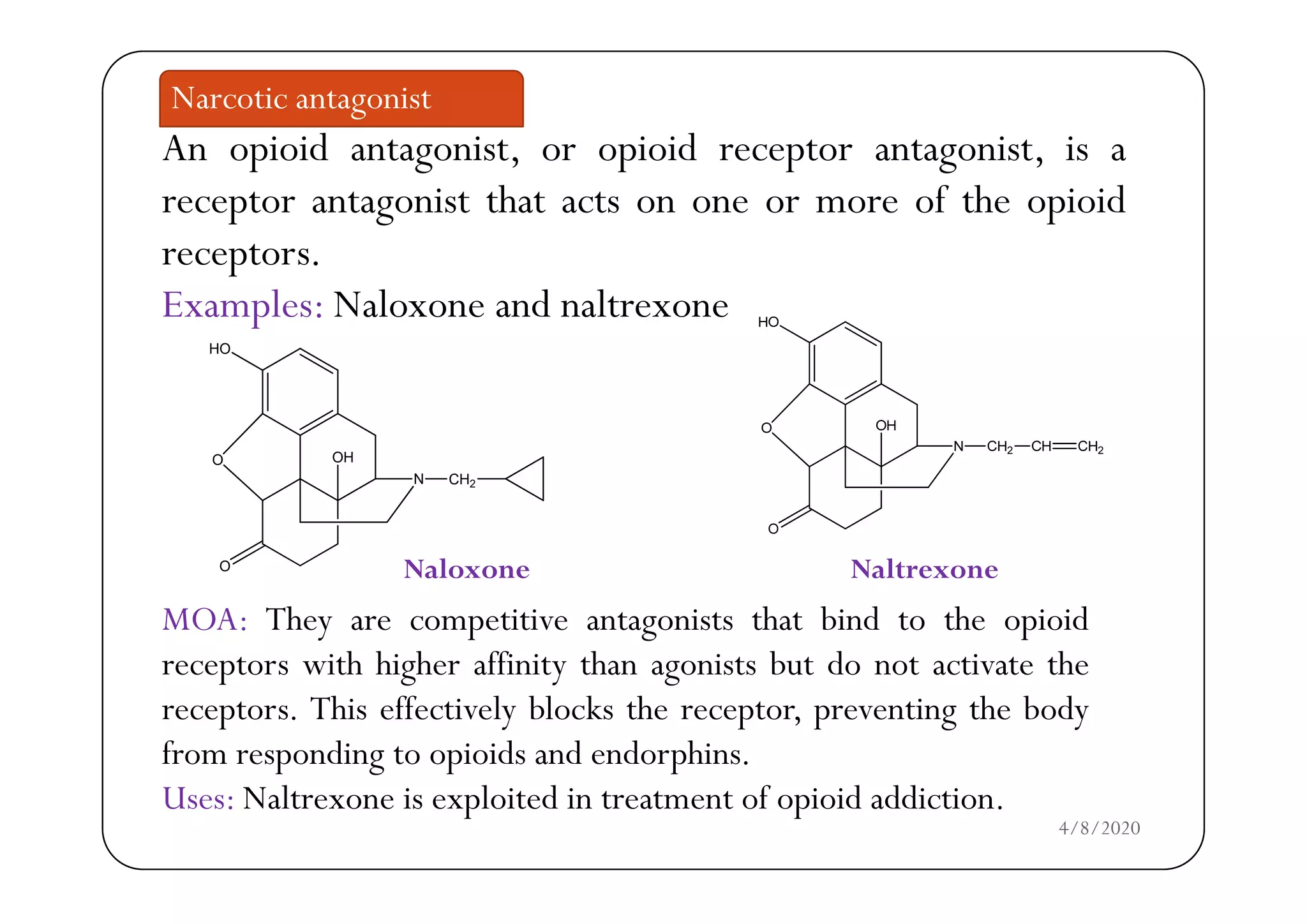
The document discusses narcotic analgesics, which are drugs that relieve severe pain by acting on the central nervous system. It categorizes these drugs into various classifications, such as morphine analogues and 4-phenylpiperidine analogues, and details their mechanisms of action and structural activity relationships. Additionally, it provides information on the uses and applications of specific narcotic analgesics like morphine, methadone, and fentanyl, alongside their pharmacological properties.