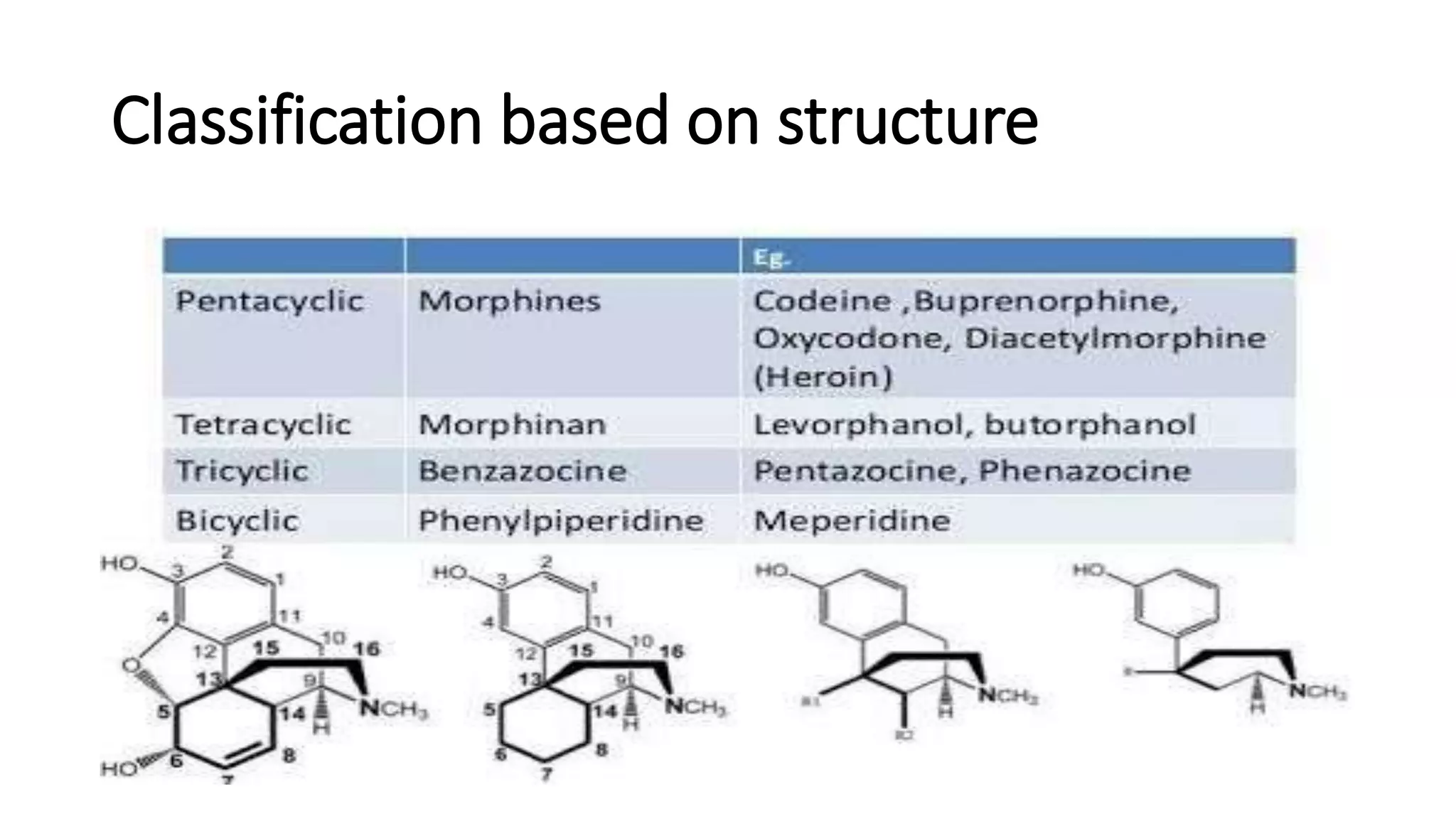
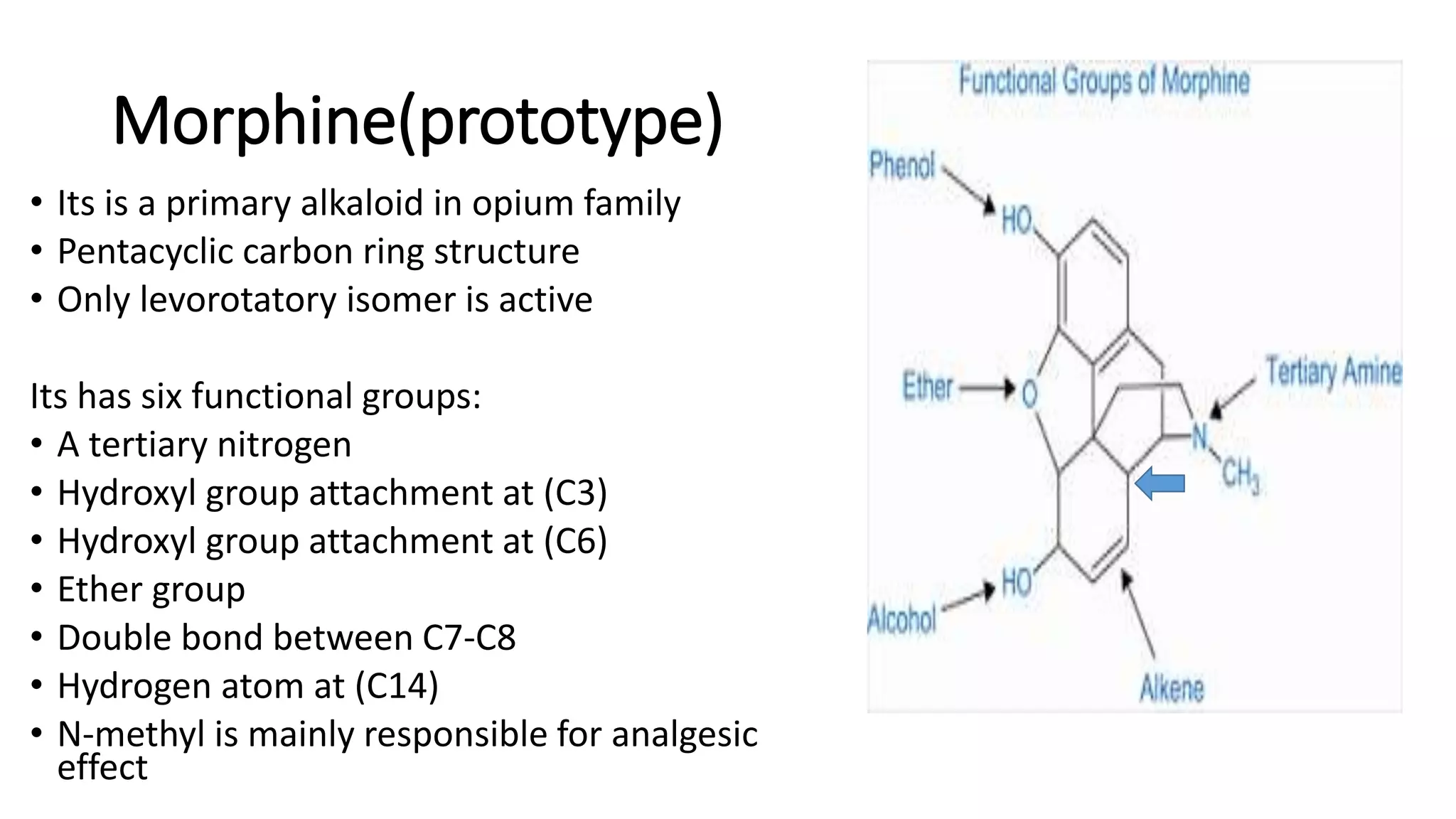
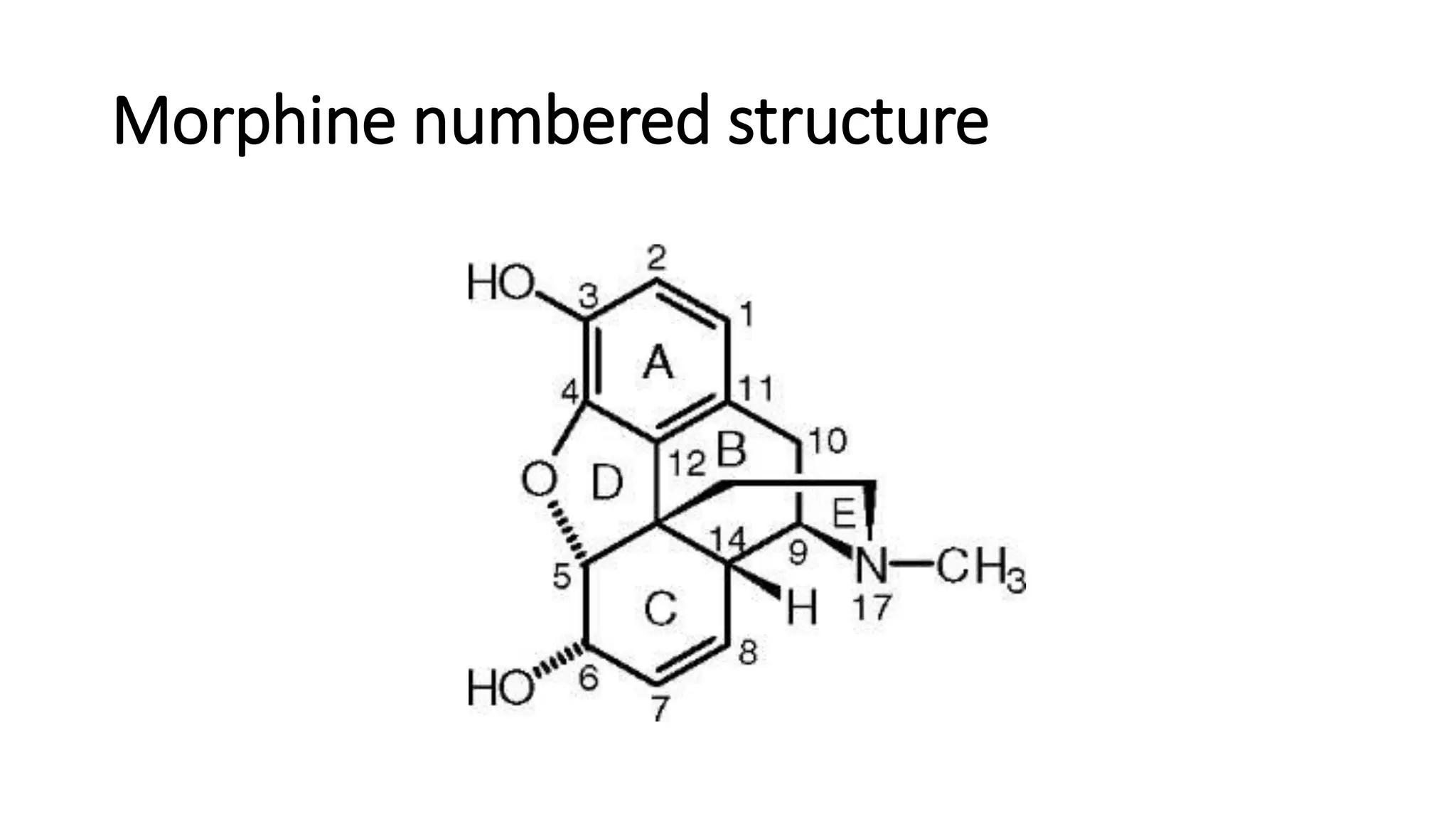
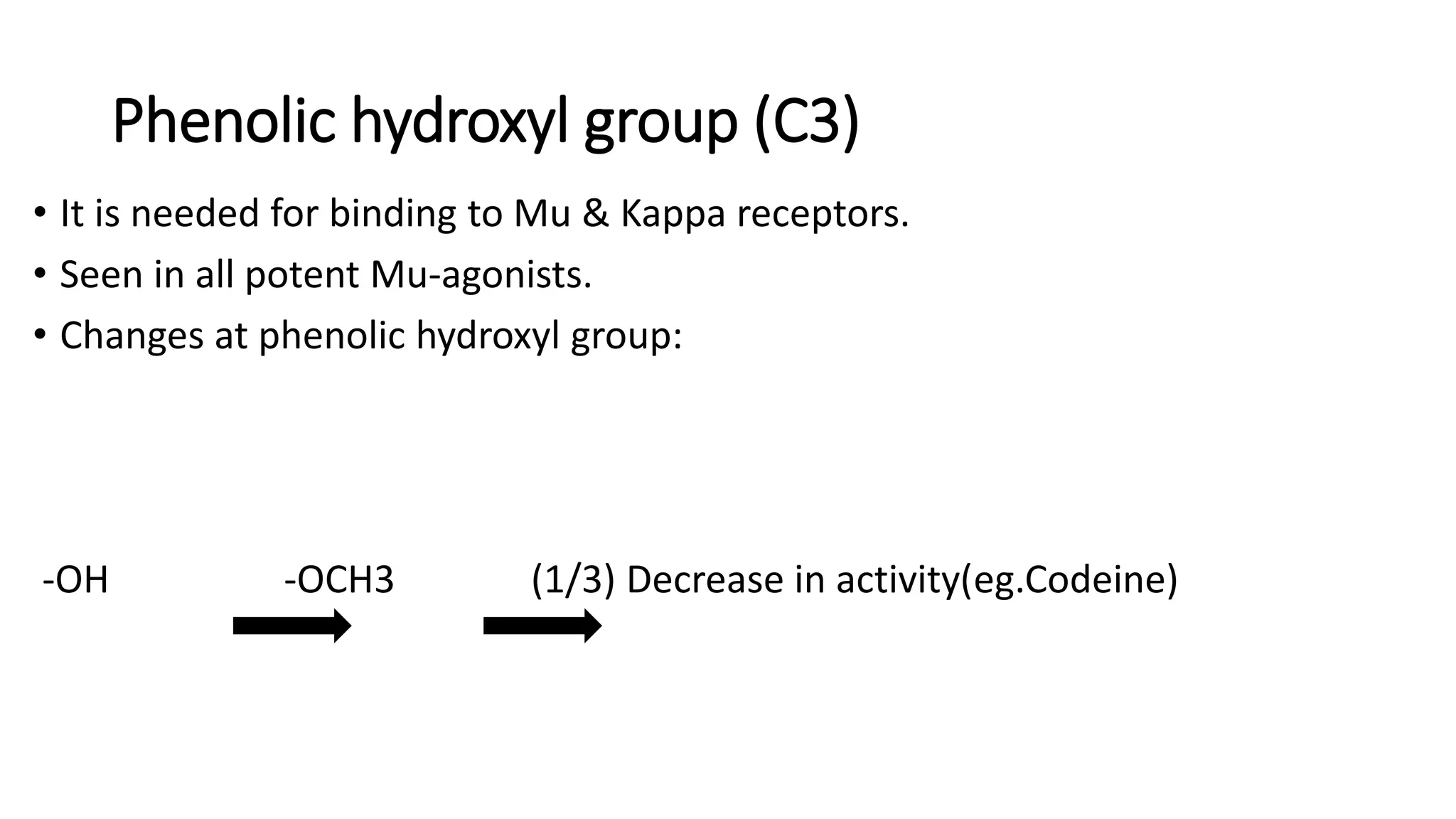
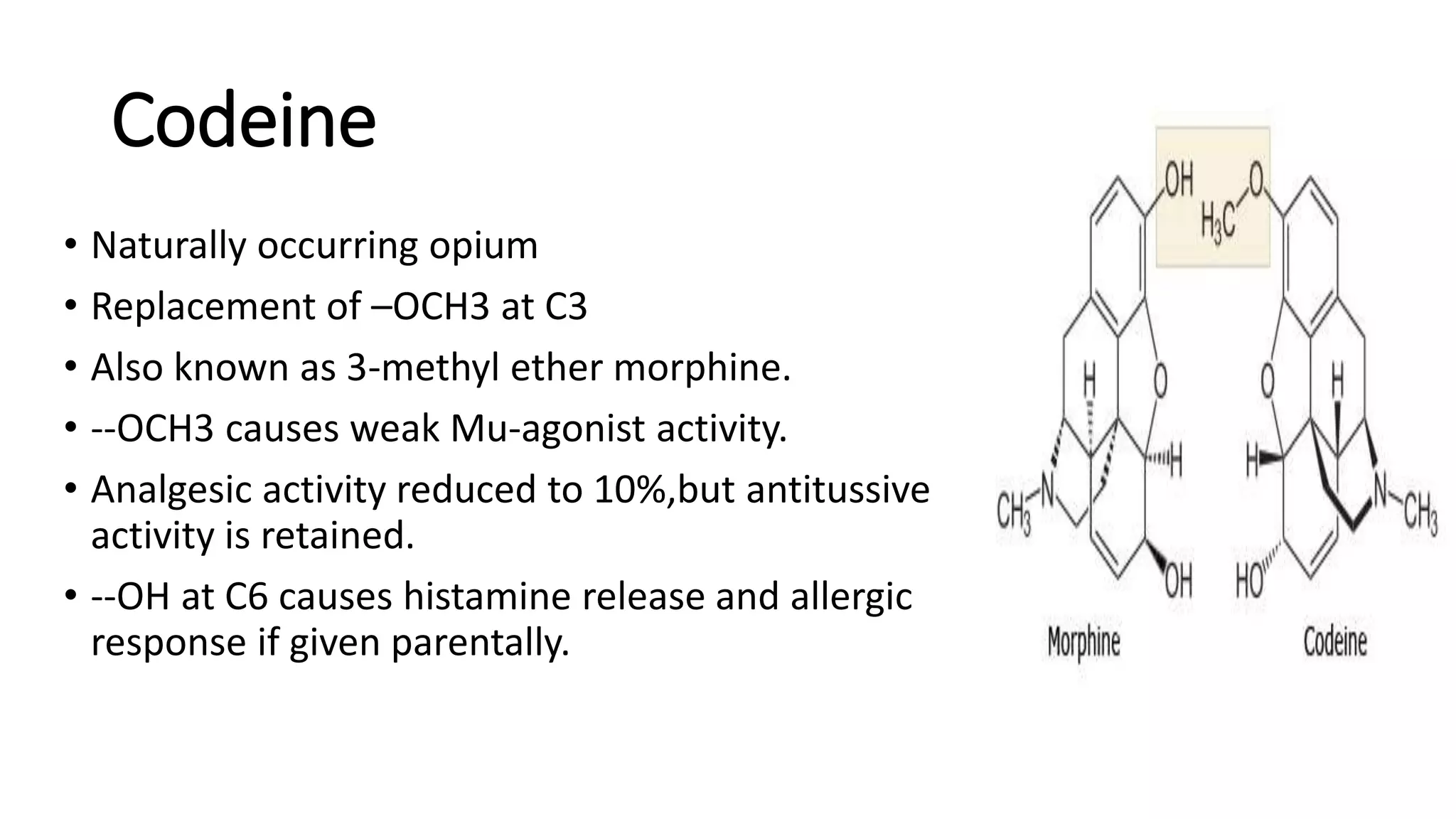
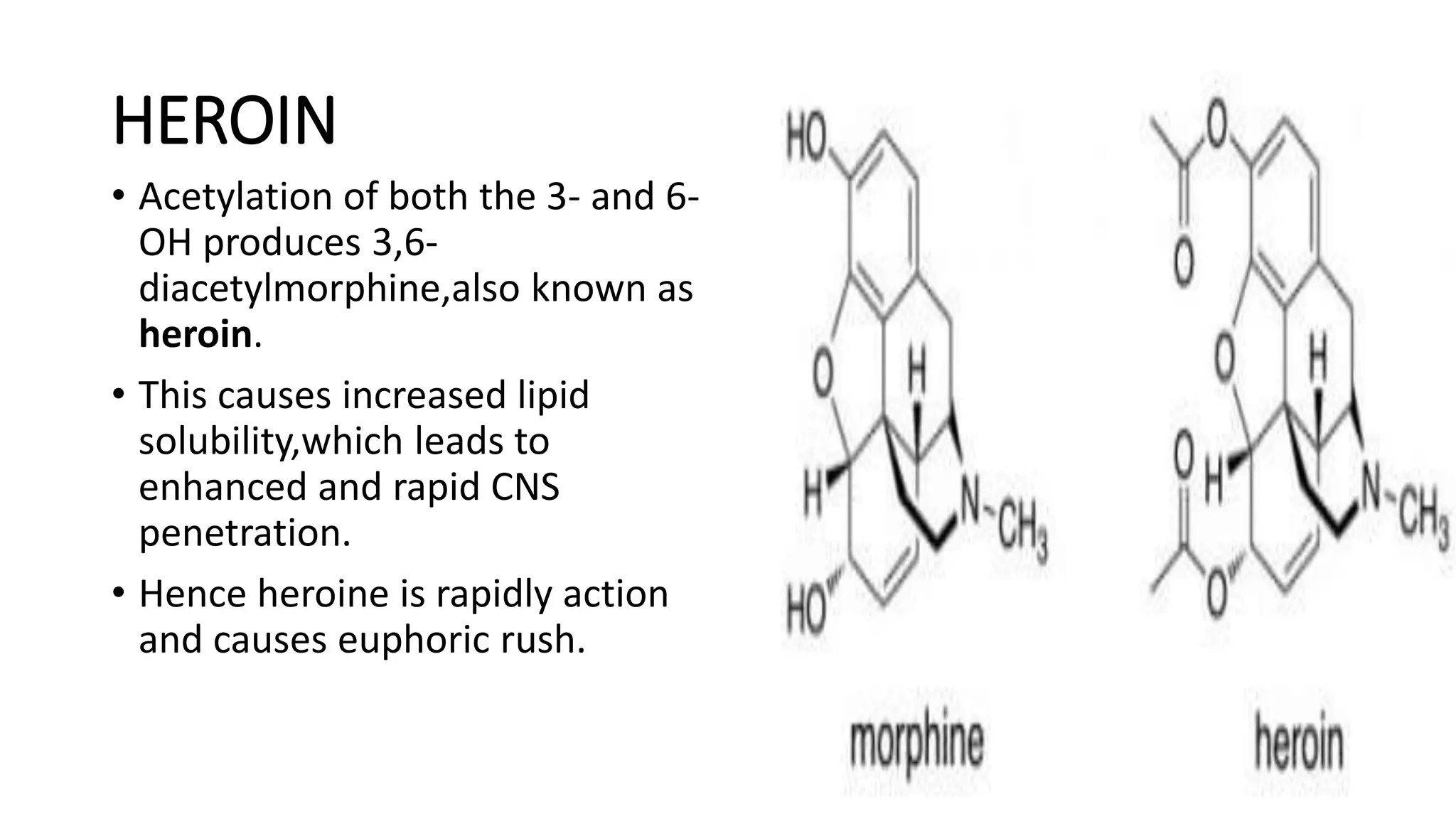
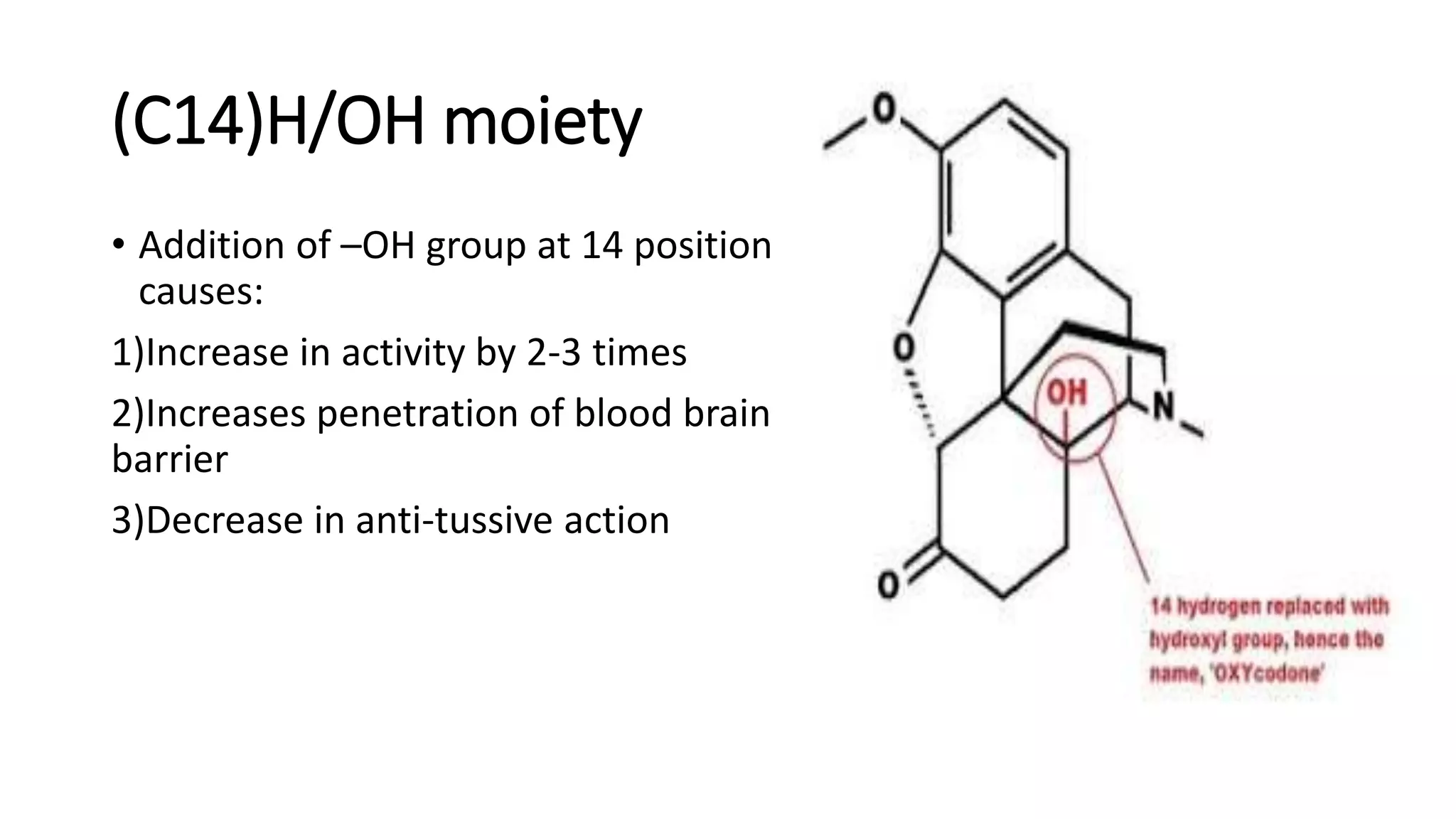
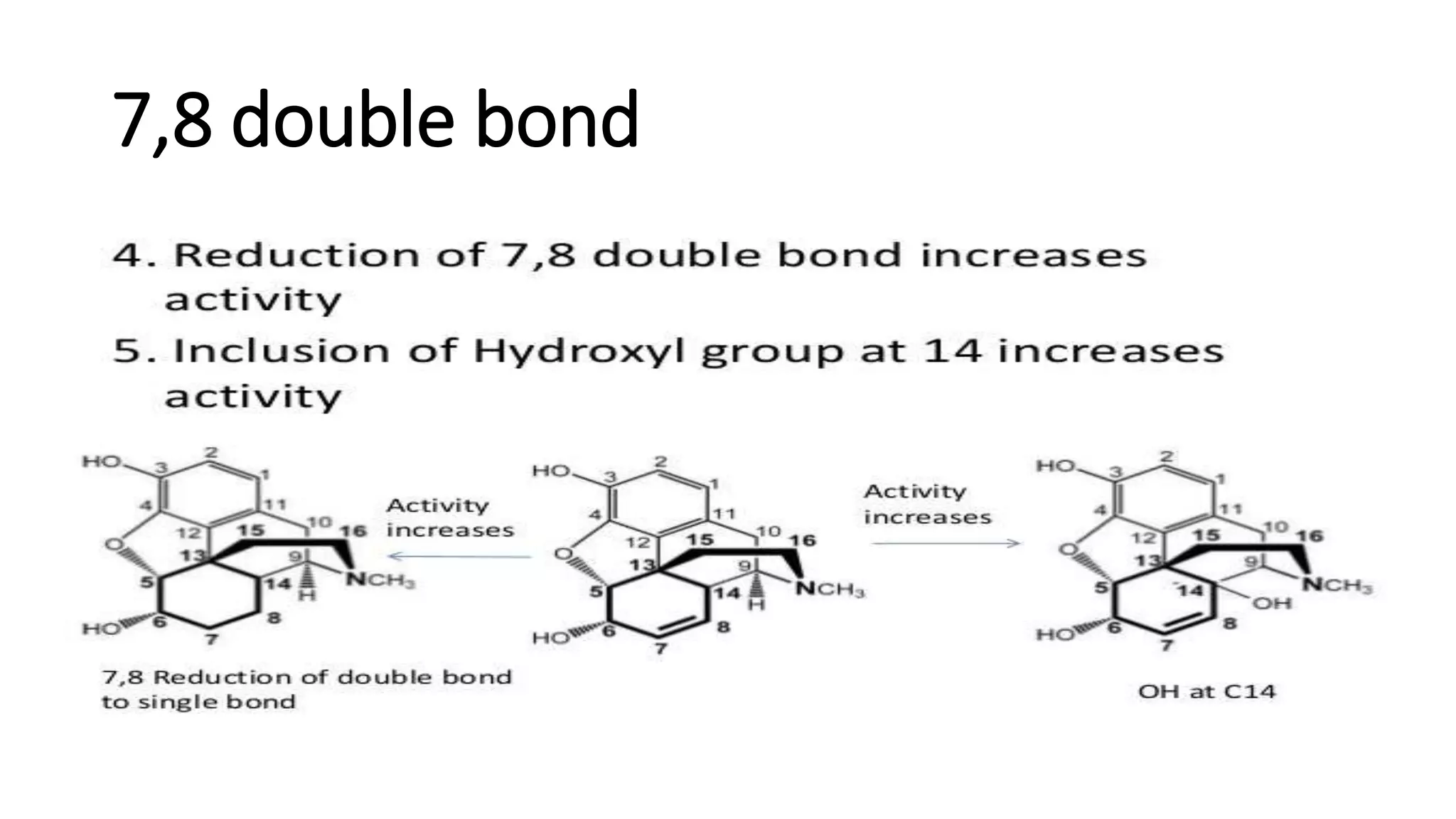
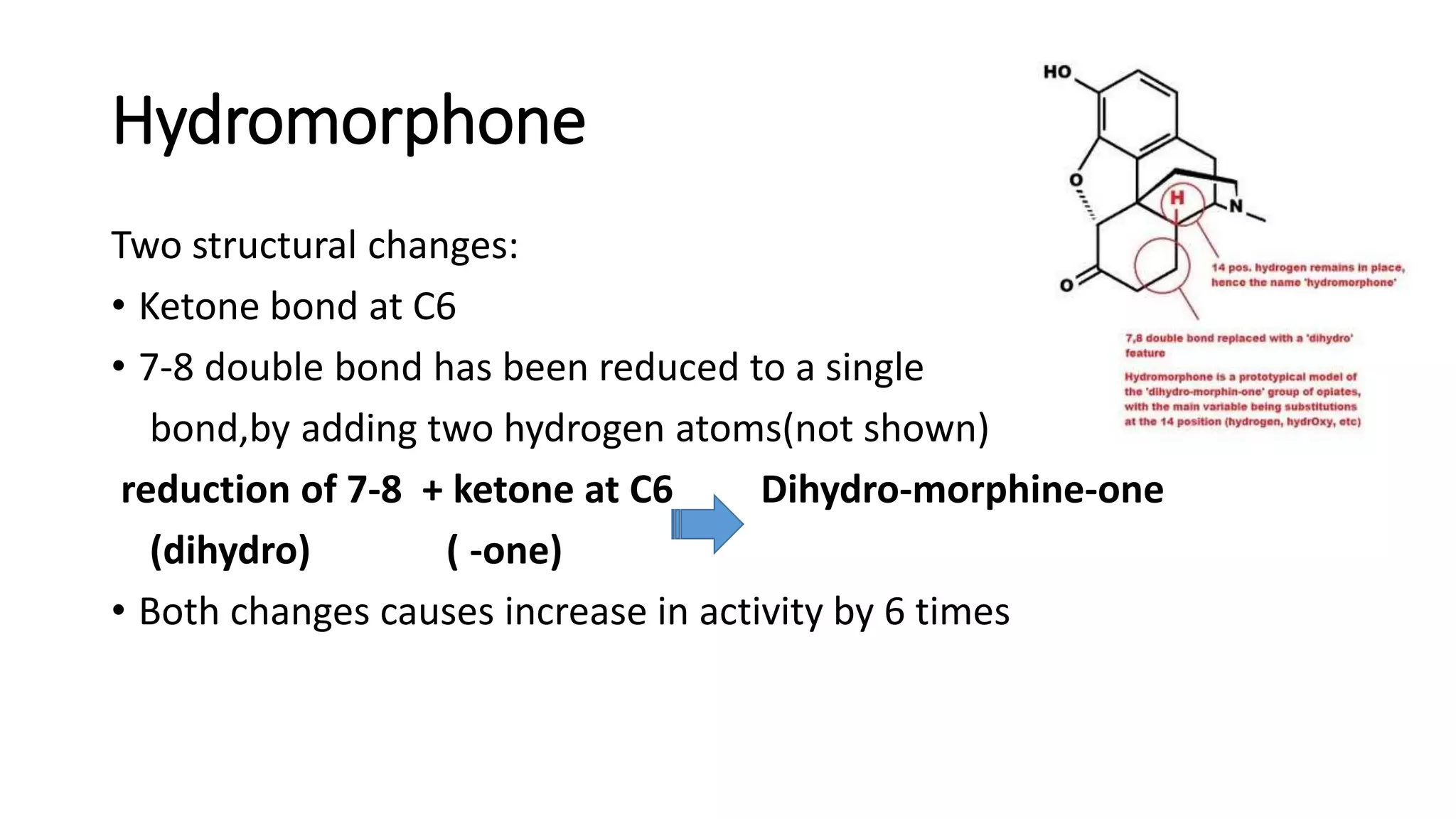
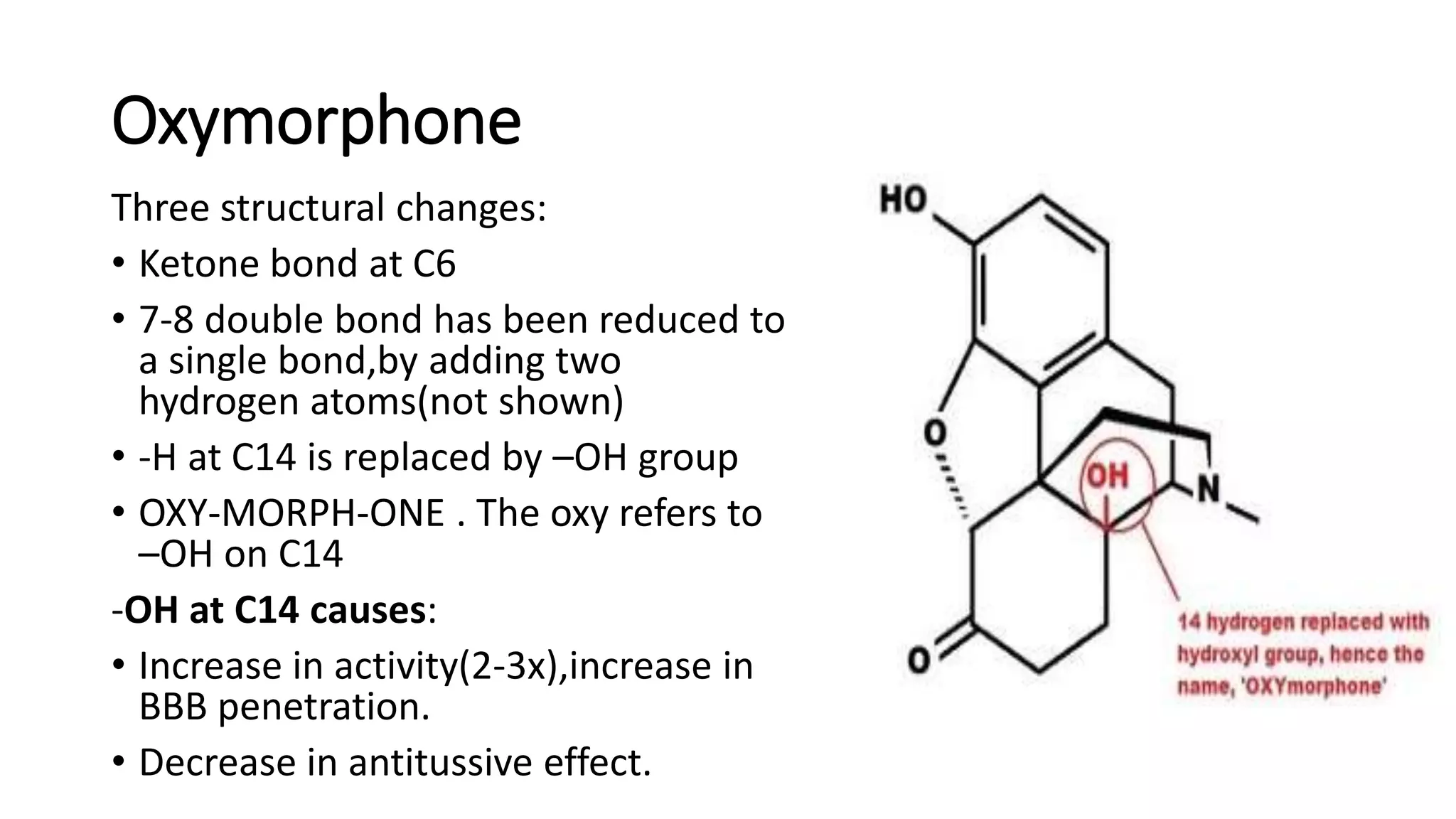
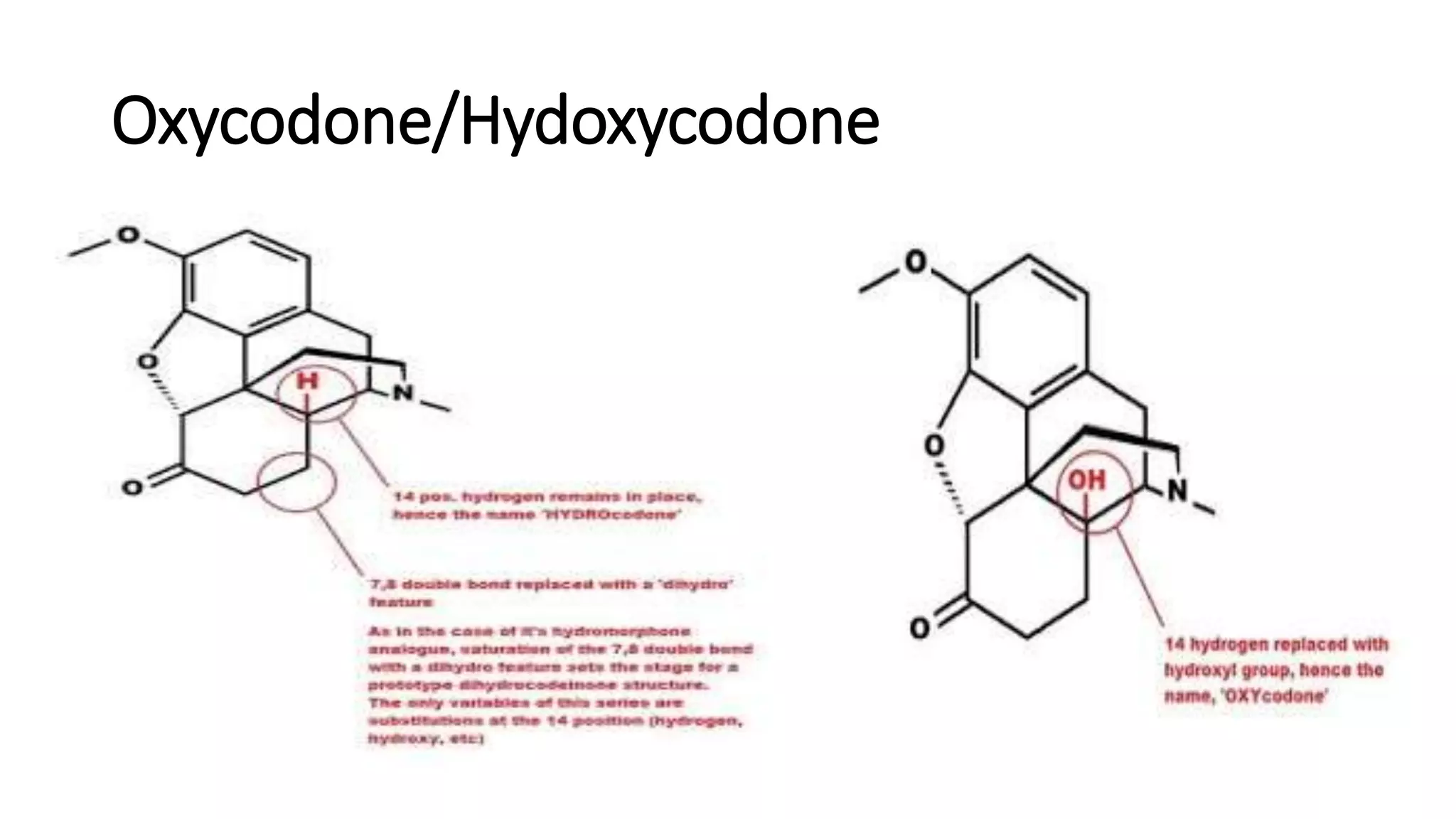
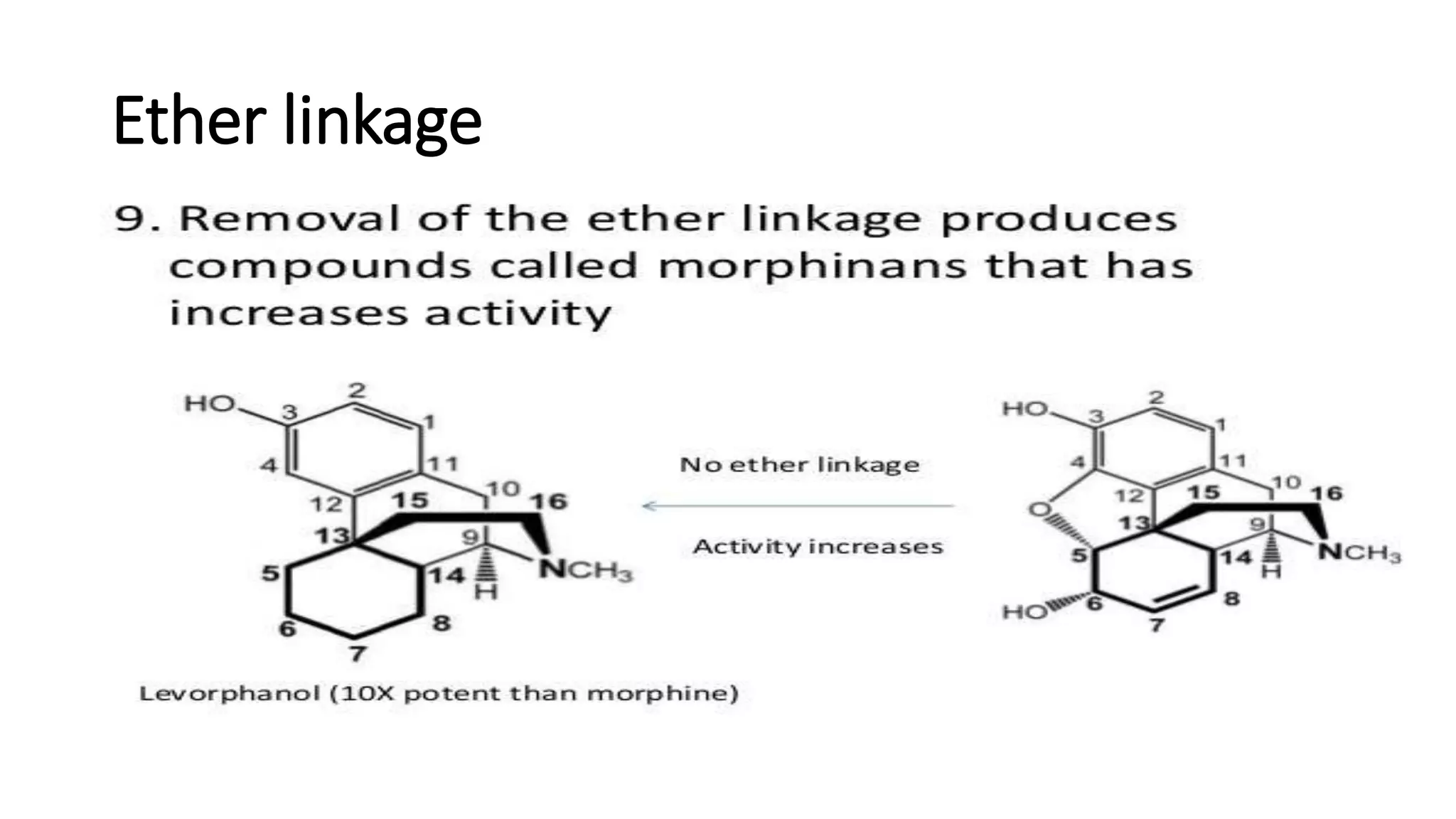
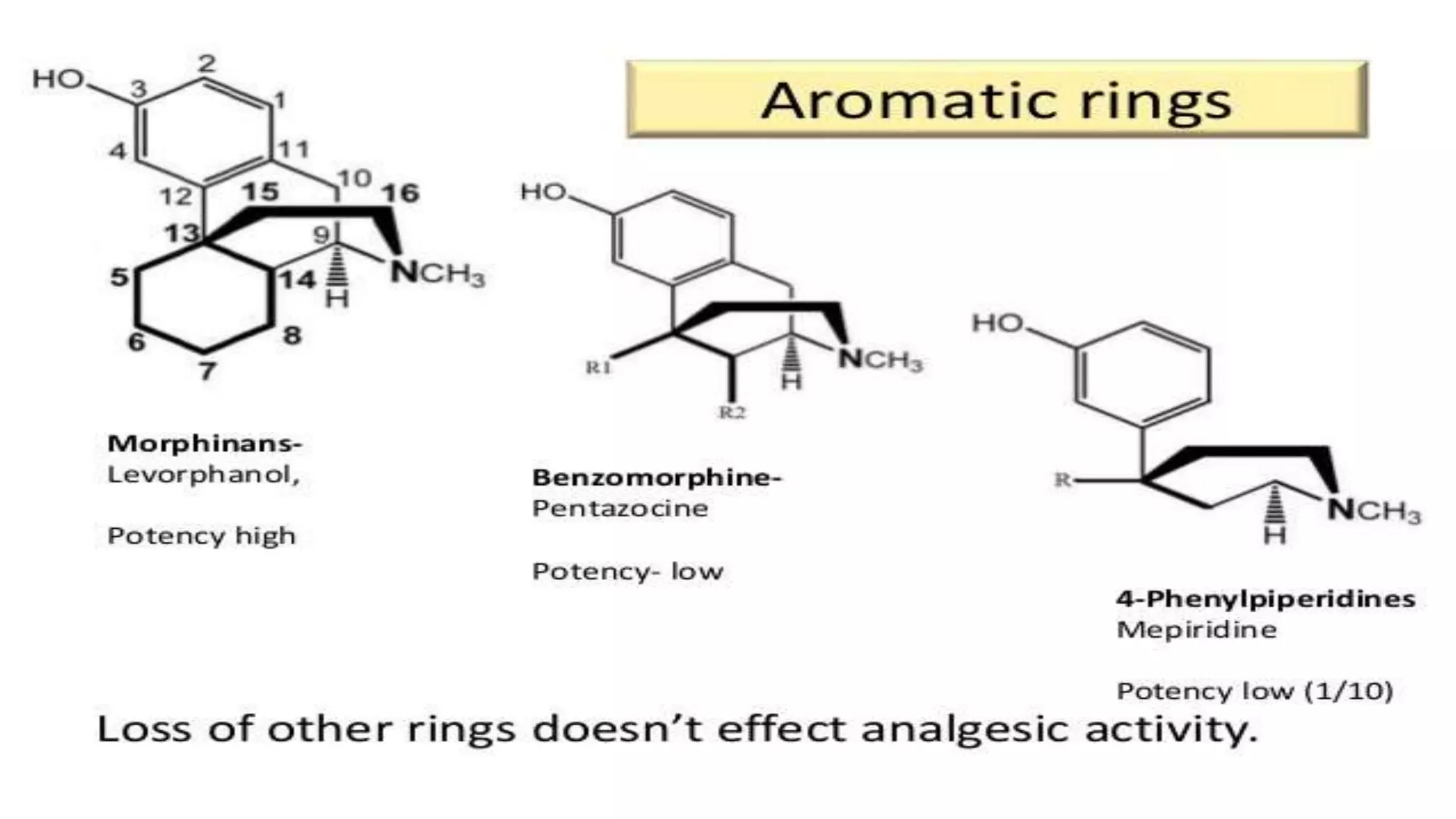
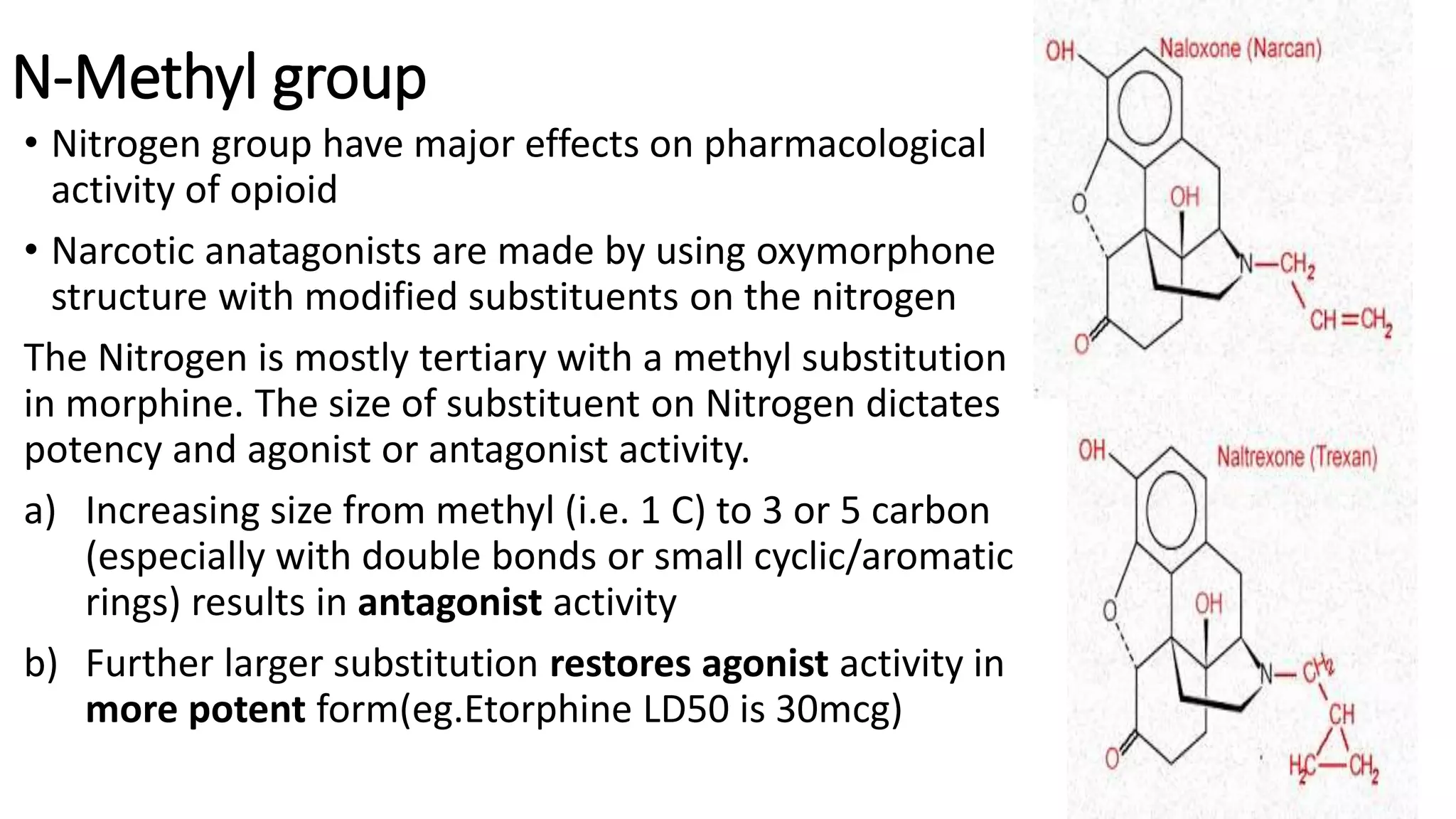
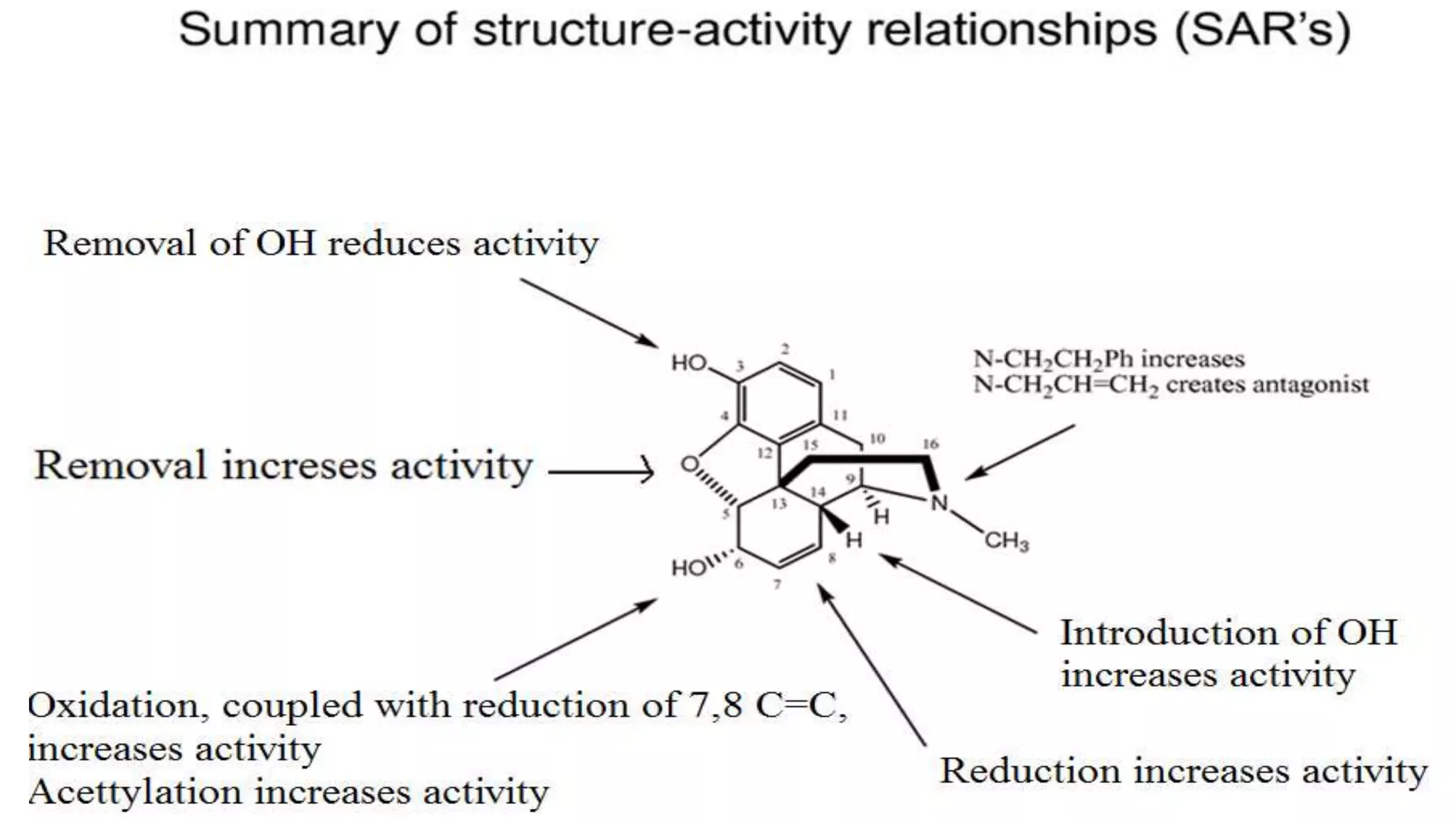
The document discusses the structure-activity relationships (SAR) of opioids, with a focus on morphine as the prototype and its various modifications. It highlights the importance of specific functional groups on the opioid molecule for efficacy and activity at mu and kappa receptors. Various derivatives such as codeine, heroin, hydromorphone, and oxymorphone are examined for their structural modifications and resulting pharmacological effects.