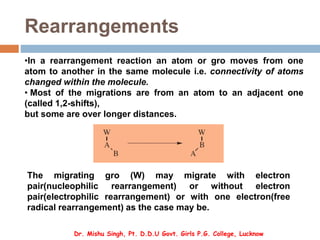
1. Molecular rearrangements involve the migration of an atom or group within the same molecule, changing the connectivity of atoms.
2. The most common migrations are 1,2-shifts of an atom or group to an adjacent atom. These shifts can be nucleophilic, electrophilic, or free radical in nature.
3. Examples of common rearrangements discussed in the document include the Wagner-Meerwein, pinacol, benzilic acid, Hofmann, Curtius, Lossen, Beckmann, and Neber rearrangements.





























































![Sommelet-Hauser
Rearrangement
•In the absence of β-carbonyl gro, the α-hydrogen is too weakly
acidic for hydroxide ion induced rearrangement.
• Thus, a strong base, such as amide ion in liquid ammonia, is to
be used, when the rearrangement takes a different course:
instead of [1,2] shift (Steven’s rearrangement), a [3,2]-sigma-
tropic rearrangement takes place which is called Sommelet-
Hauser rearrangement.
Dr. Mishu Singh, Pt. D.D.U Govt. Girls P.G. College, Lucknow](https://image.slidesharecdn.com/molecular-rearrangements-pps-230318081910-7429ea33/85/molecular-rearrangements-pps-ppt-63-320.jpg)


![Wittig Rearrangement
Ethers undergo [1,2]-sigmatropic rearrangement in the presence
of strong base such as amide ion or phenyllithium to give more
stable oxyanion.
The mechanism is analogous to that of Stevens rearrangement
Dr. Mishu Singh, Pt. D.D.U Govt. Girls P.G. College, Lucknow](https://image.slidesharecdn.com/molecular-rearrangements-pps-230318081910-7429ea33/85/molecular-rearrangements-pps-ppt-66-320.jpg)










![Claisen Rearrangement
Aryl allyl ethers undergo [3,3]-sigma-tropic
rearrangement on being heating to allyl-phenols
Dr. Mishu Singh, Pt. D.D.U Govt. Girls P.G. College, Lucknow](https://image.slidesharecdn.com/molecular-rearrangements-pps-230318081910-7429ea33/85/molecular-rearrangements-pps-ppt-77-320.jpg)


