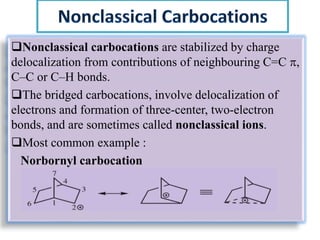
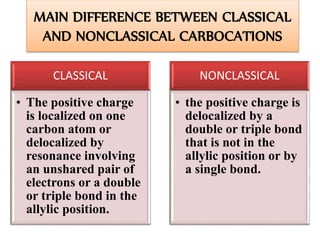
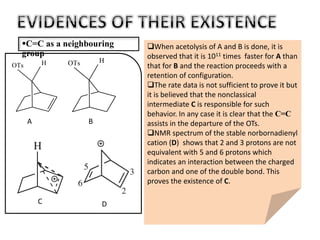
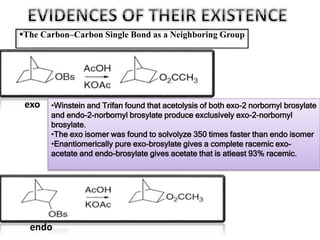
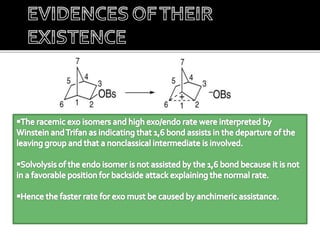
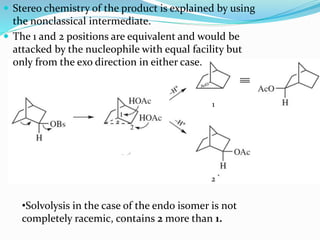
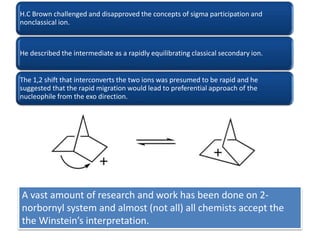
This document discusses classical and nonclassical carbocations. Nonclassical carbocations have charge delocalization from neighboring bonds like C=C pi bonds. The main difference is that classical carbocations have charge localized on one carbon, while nonclassical carbocations have charge delocalized by double or single bonds not in the allylic position. Examples like the norbornyl carbocation are given to show how neighboring double bonds can stabilize and delocalize charge through 3-center bonds. Reaction rates and product stereochemistry provide evidence for nonclassical intermediates. While some challenged this view, most chemists accept nonclassical interpretations of carbocation reactions.