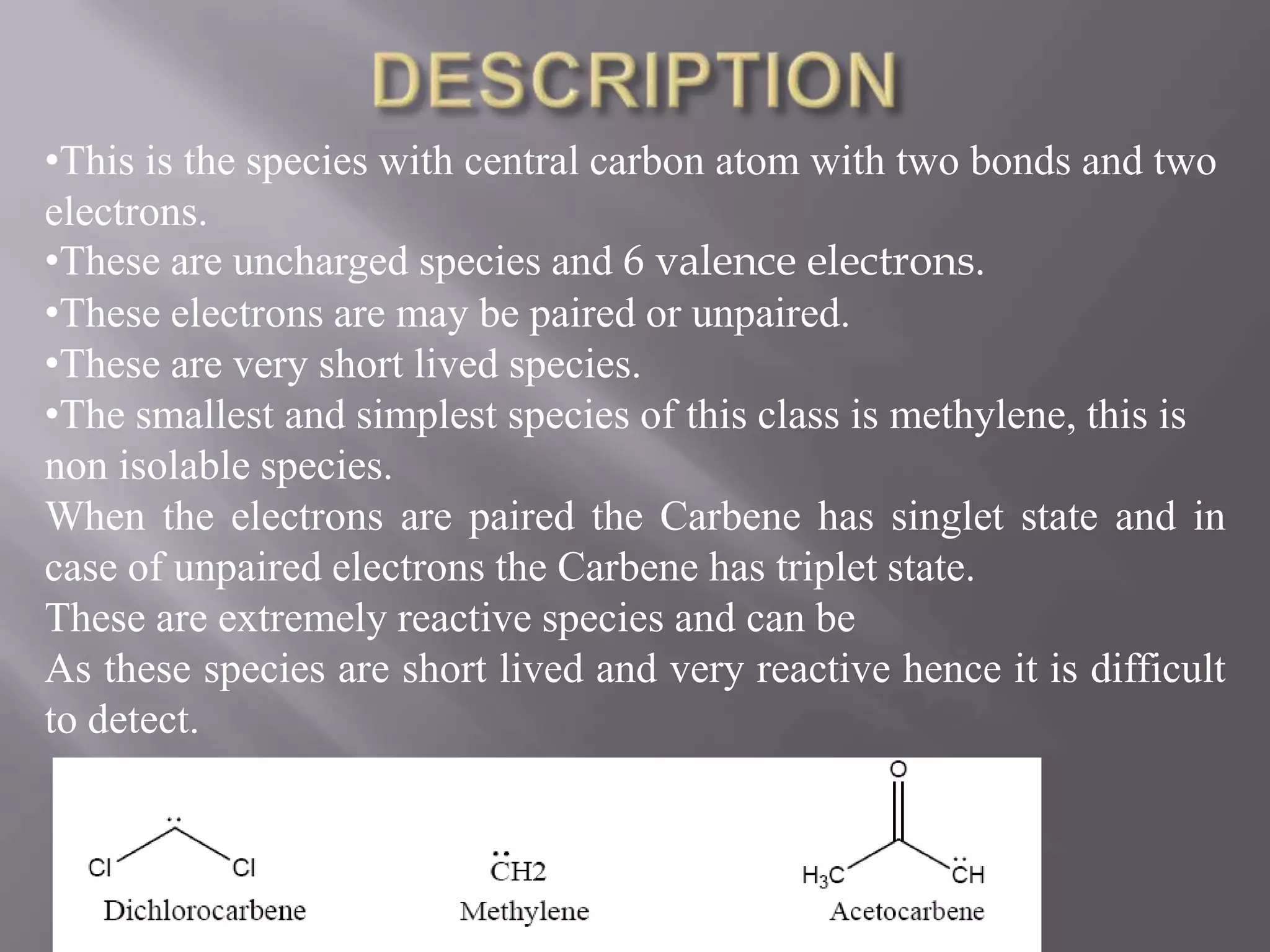
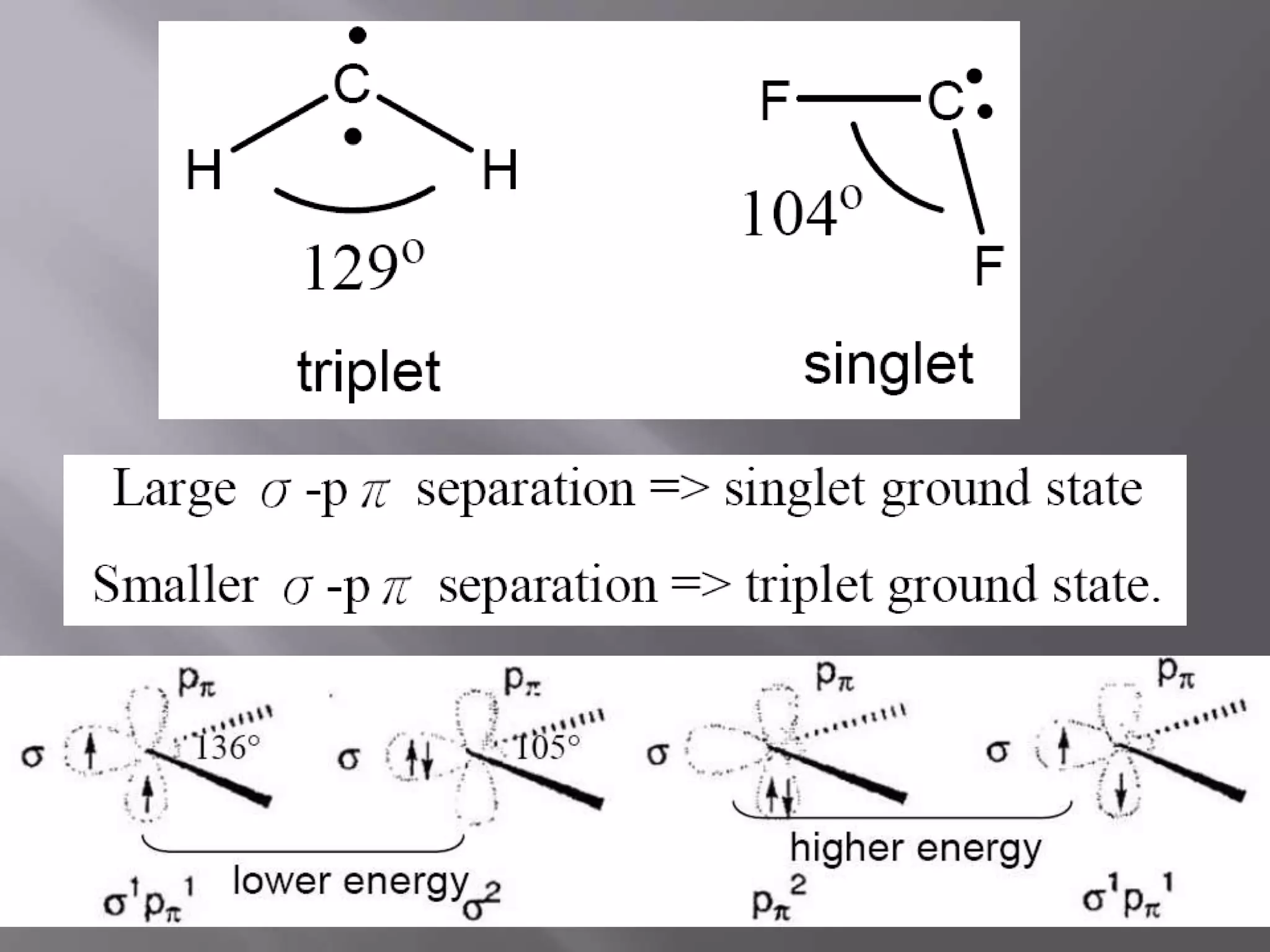
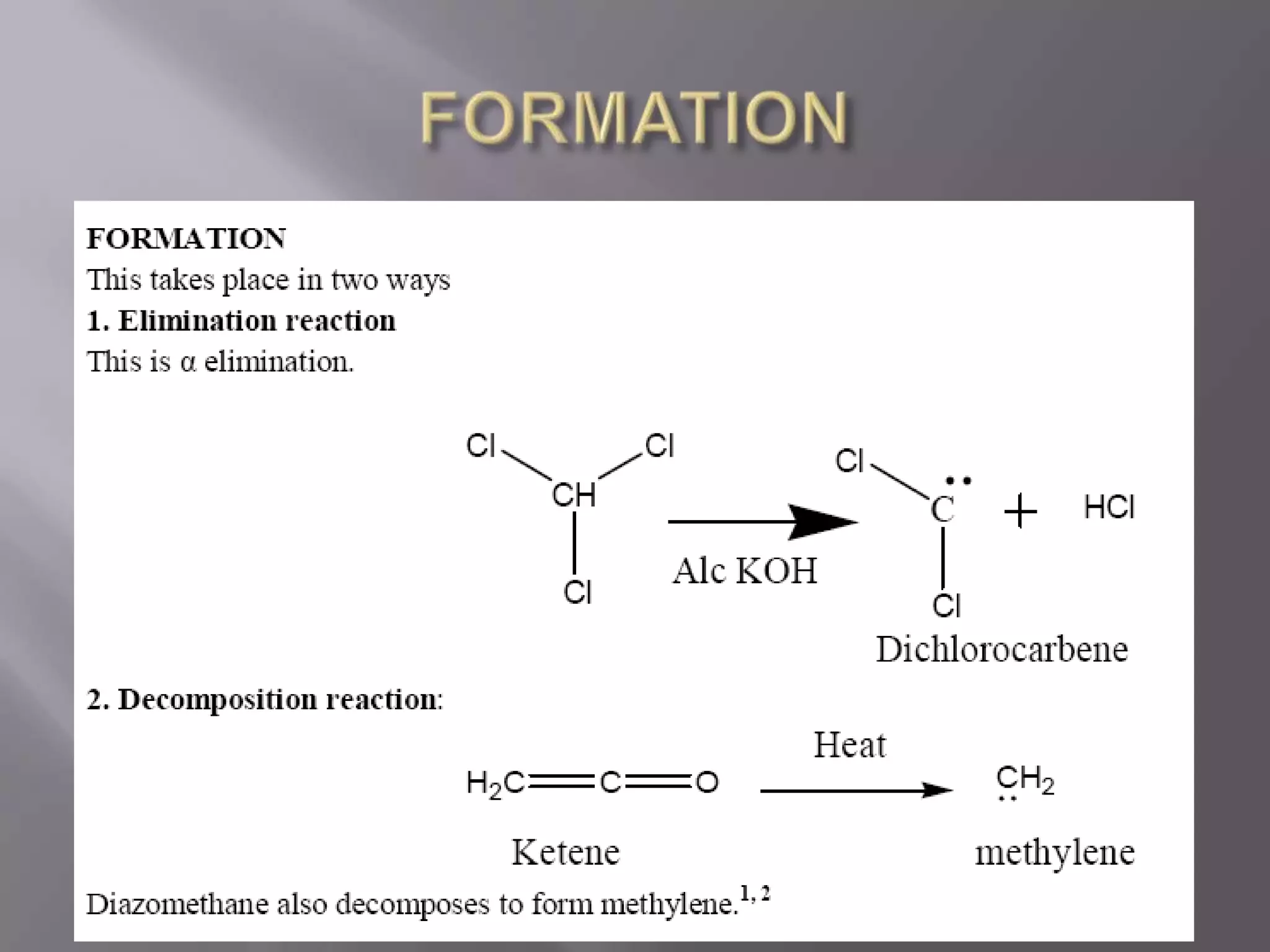
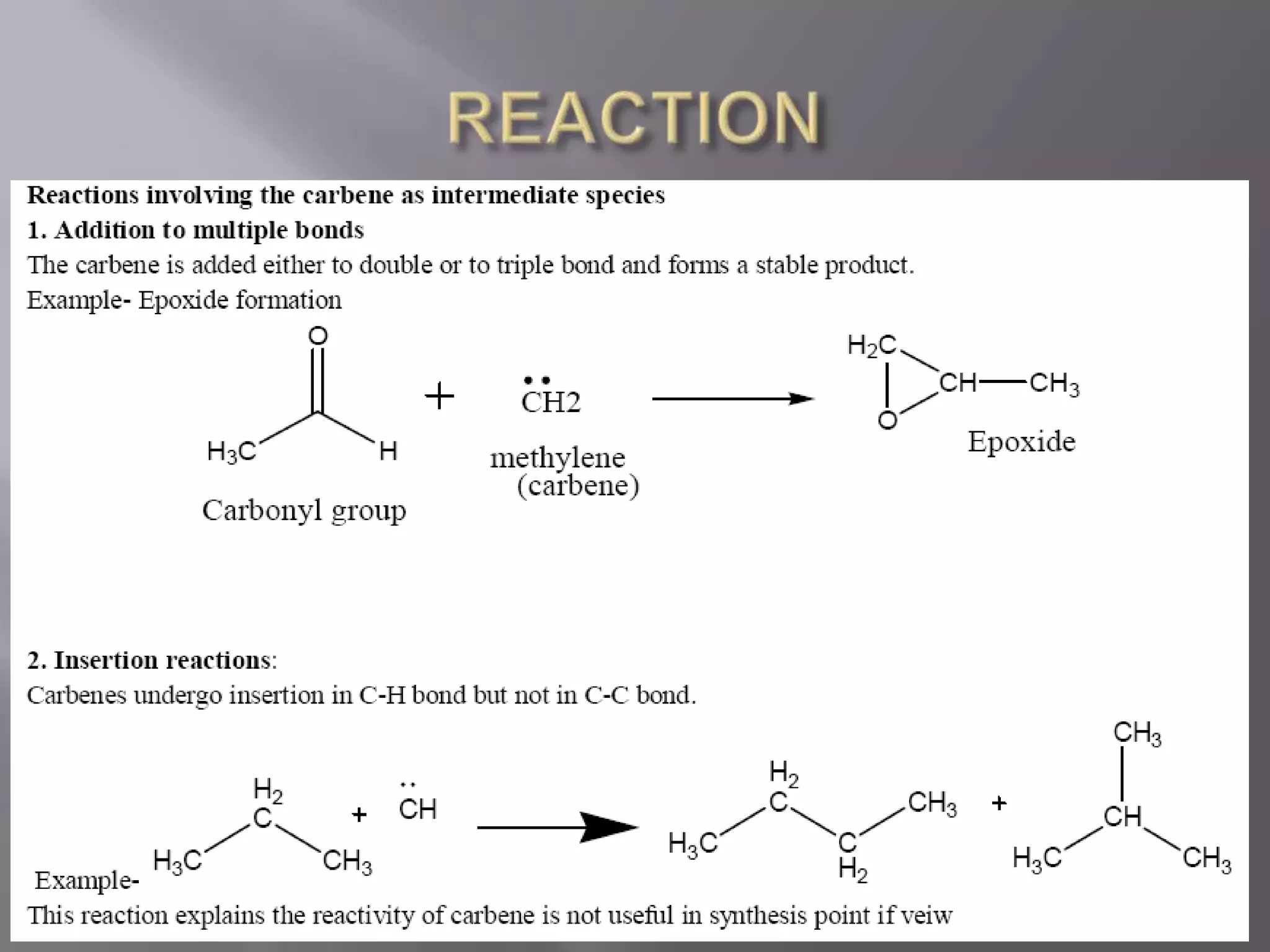
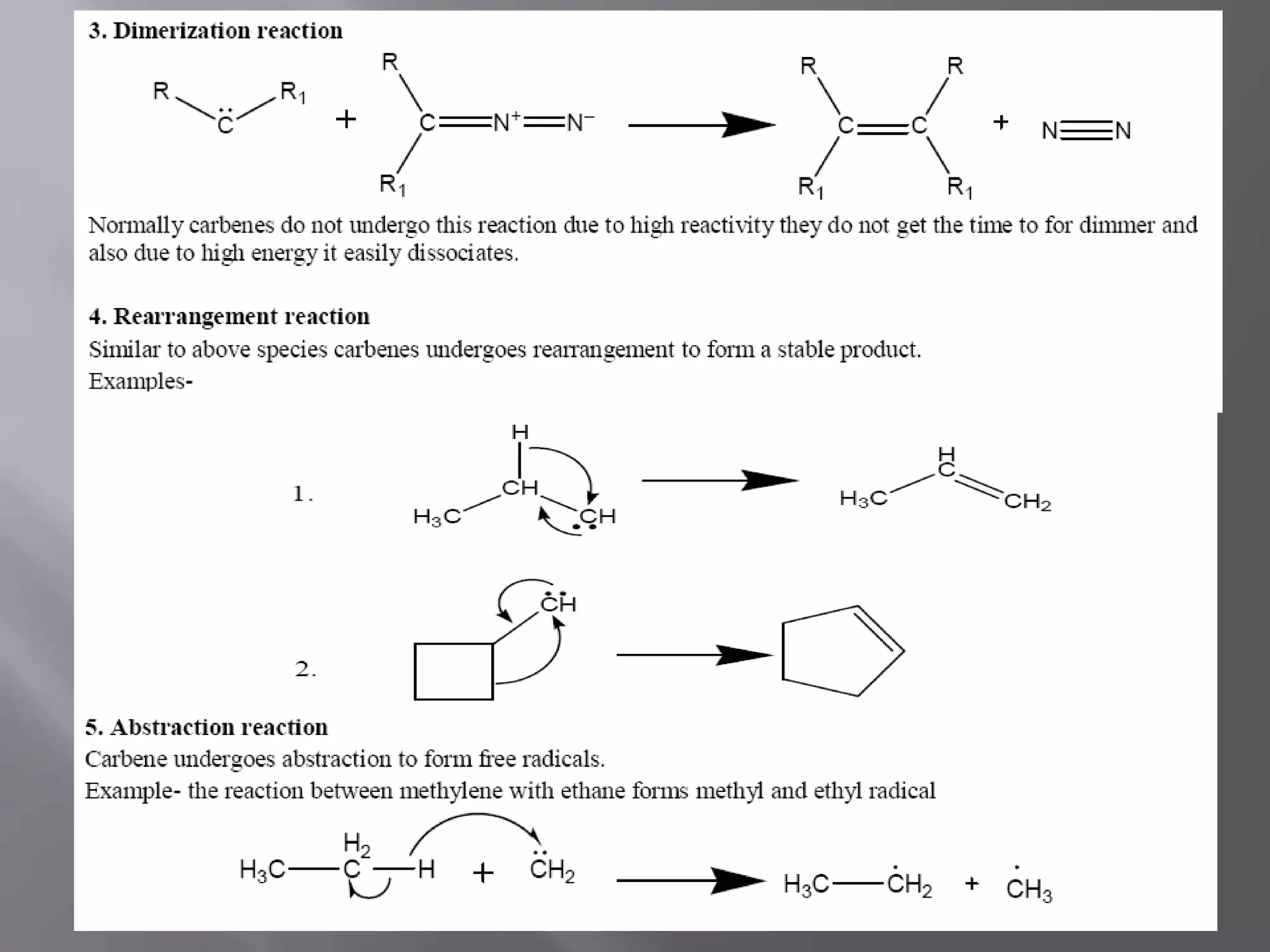
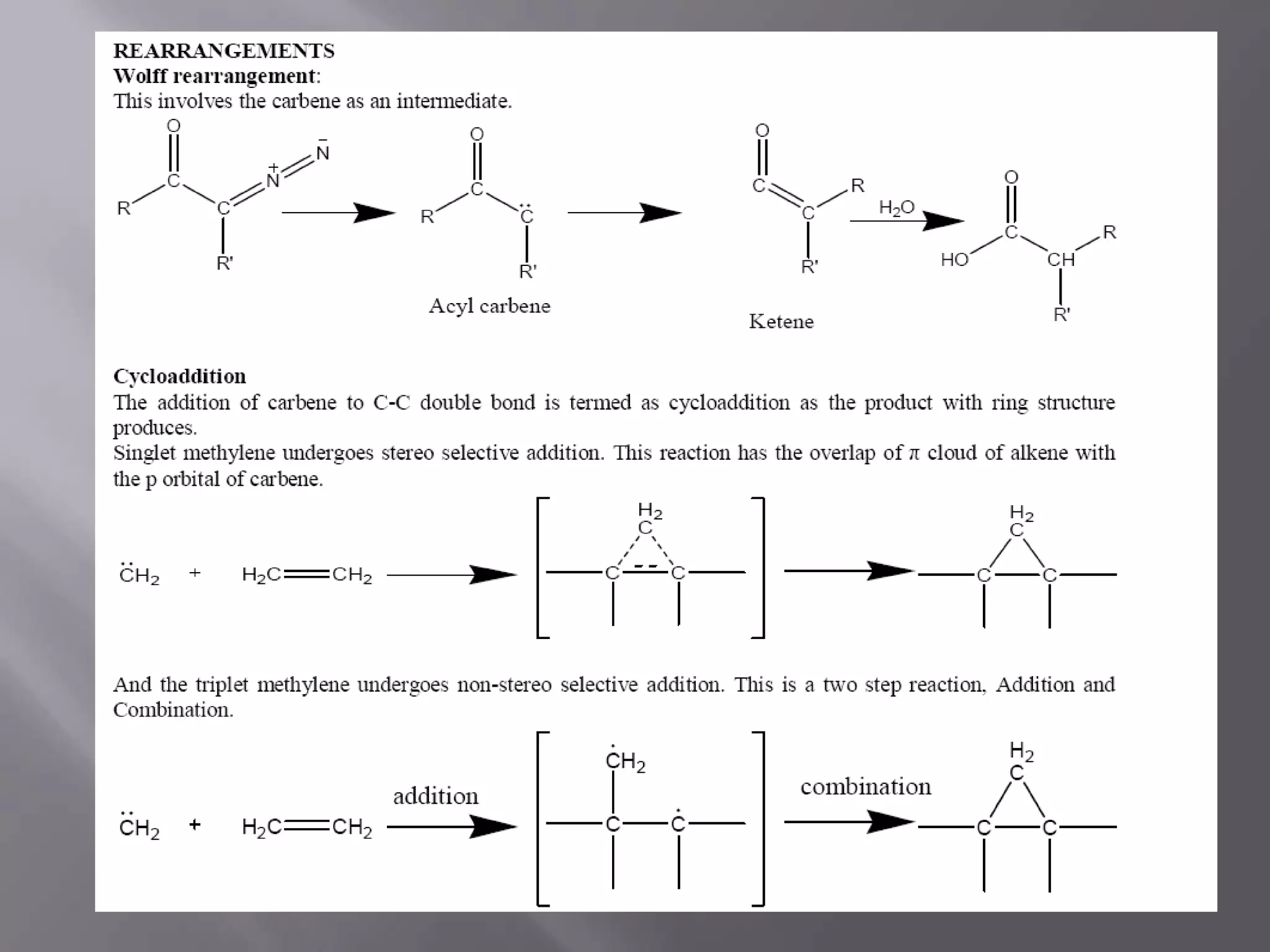
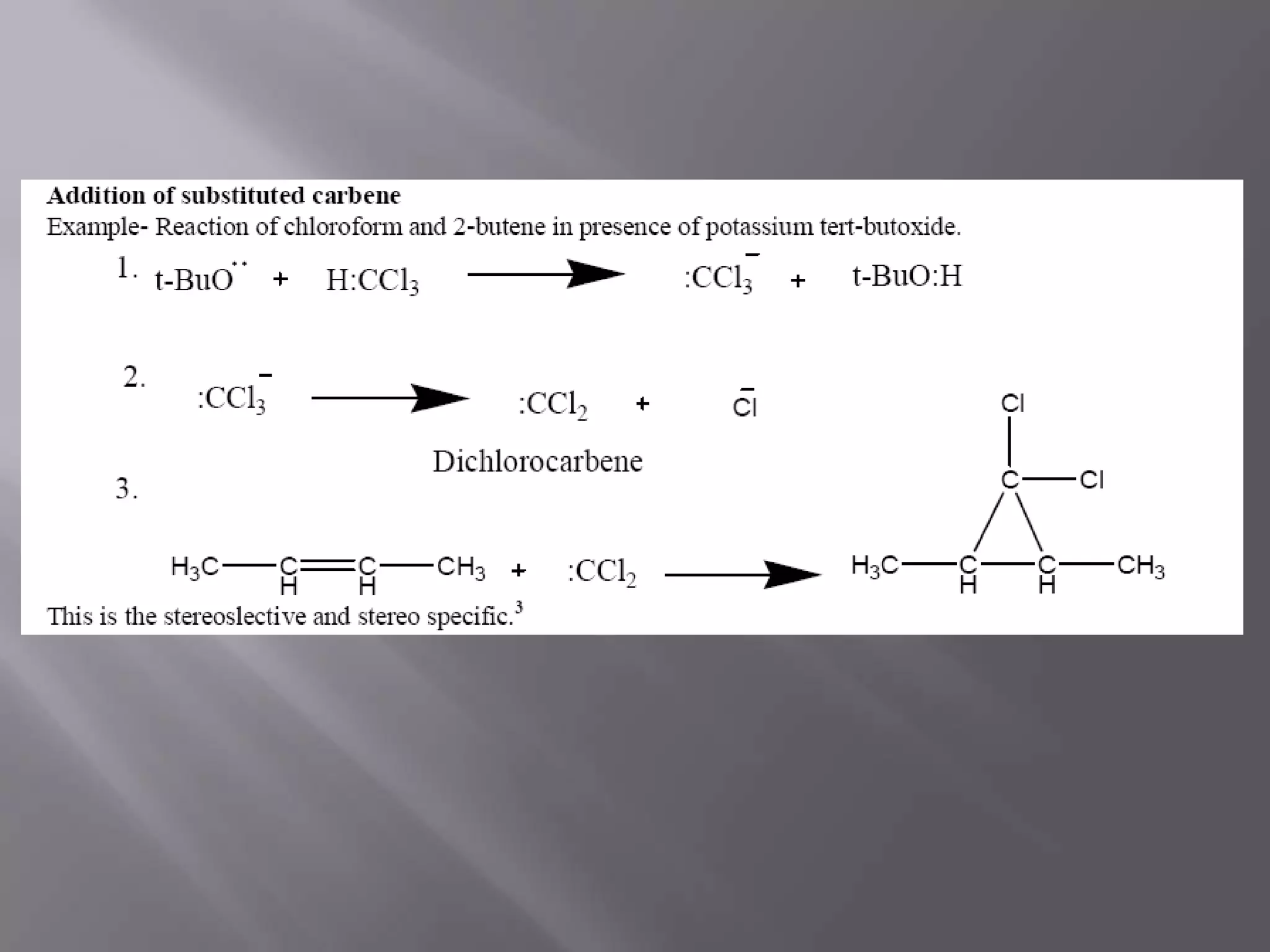
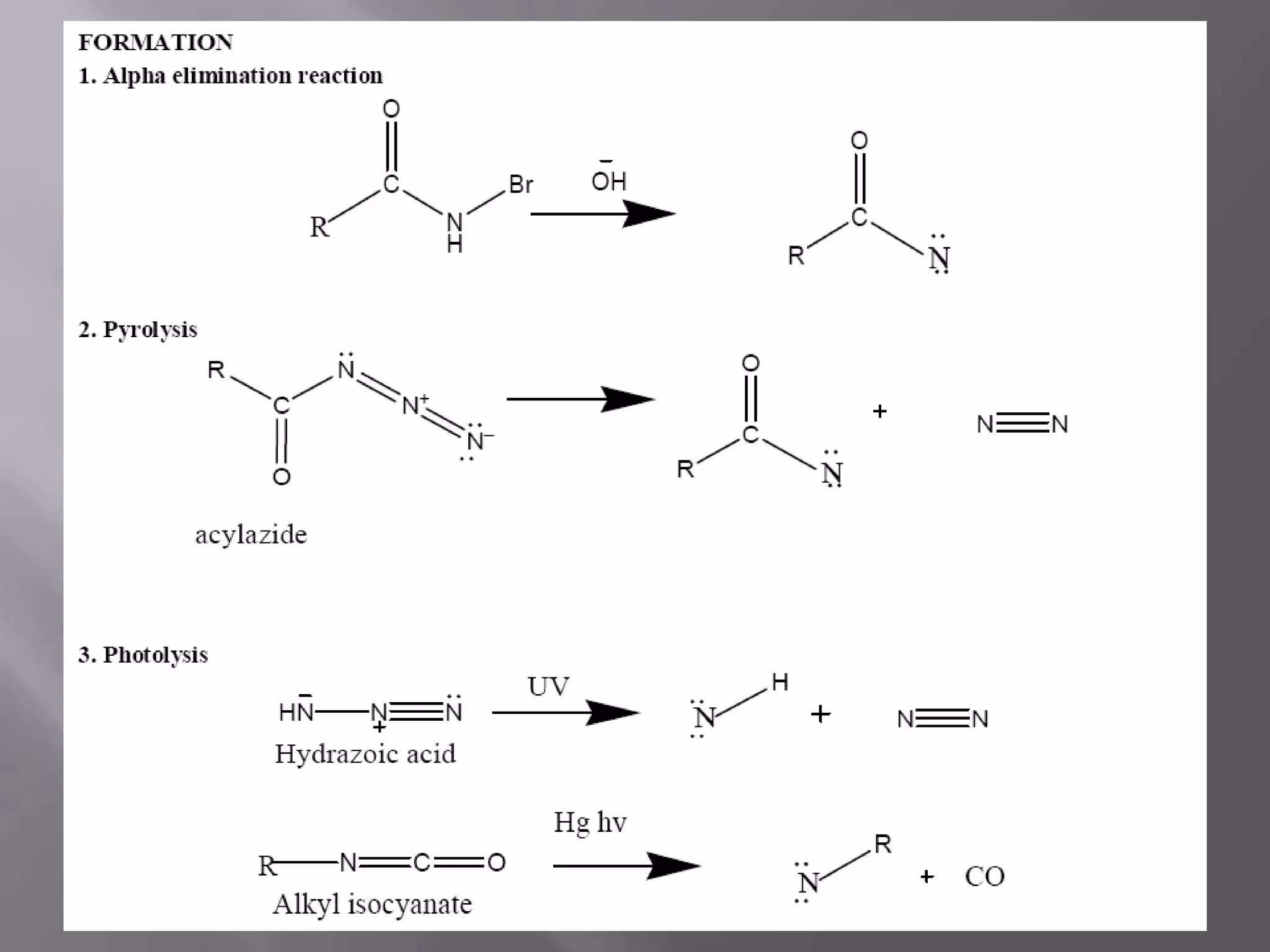
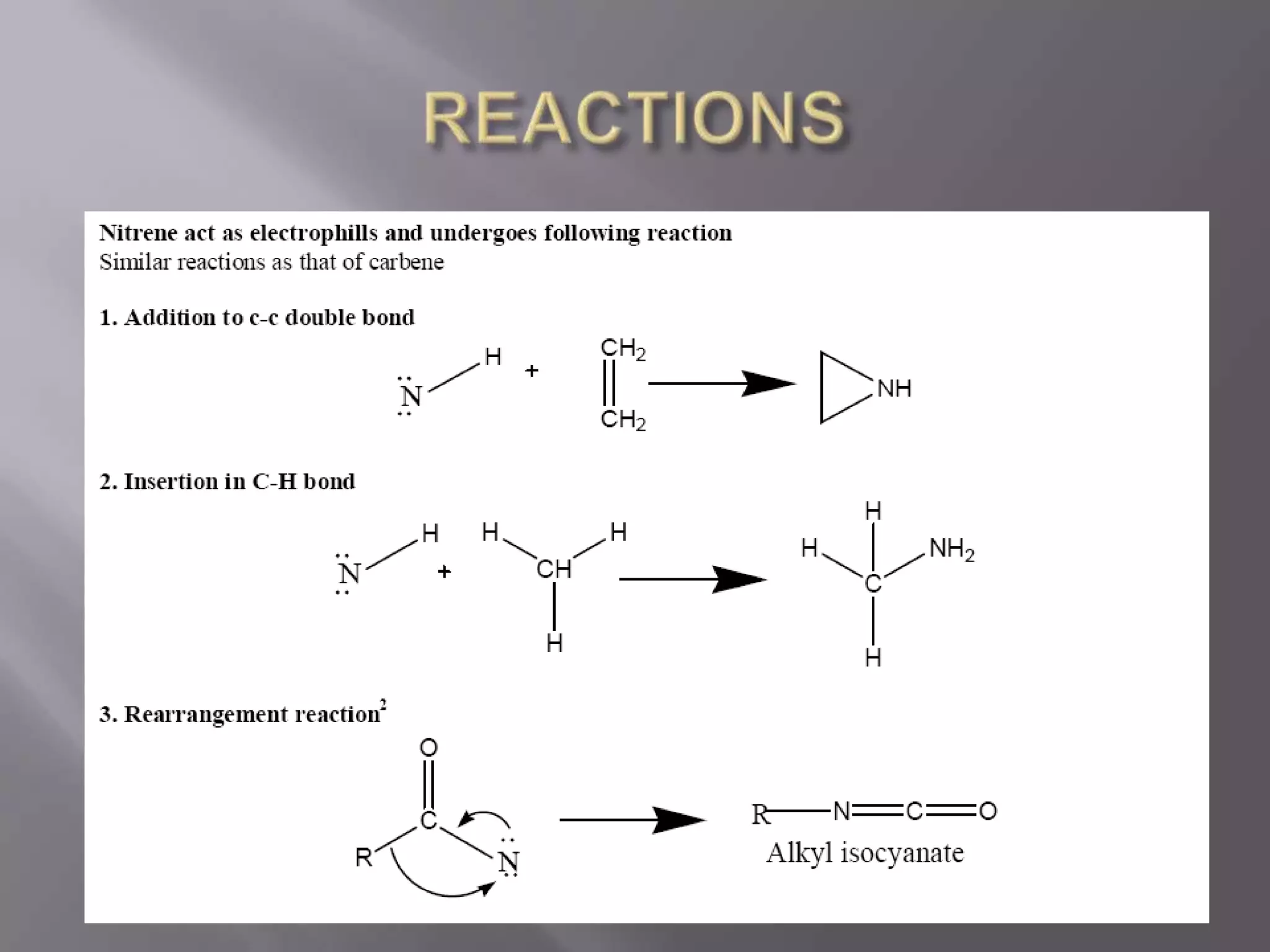
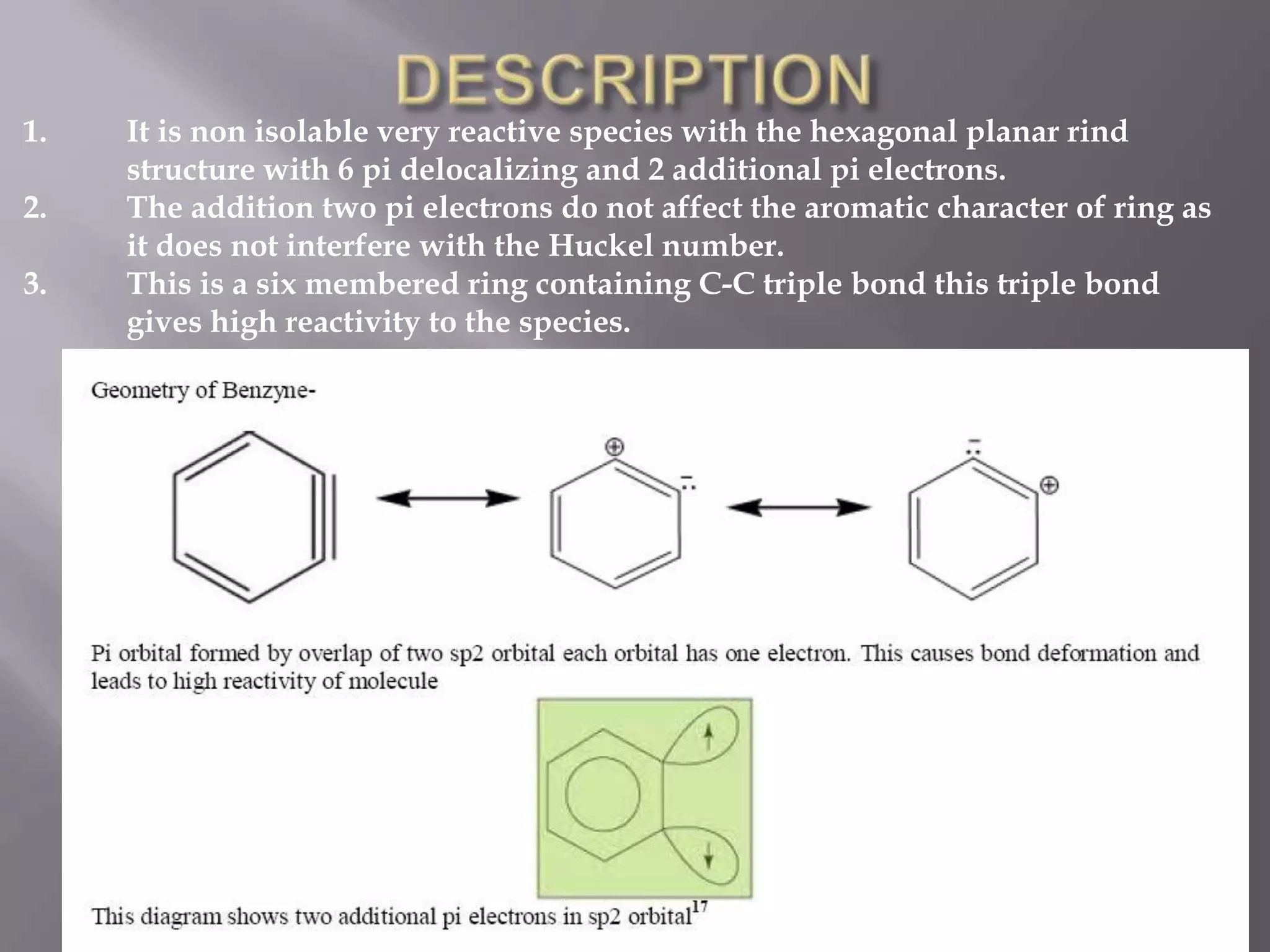
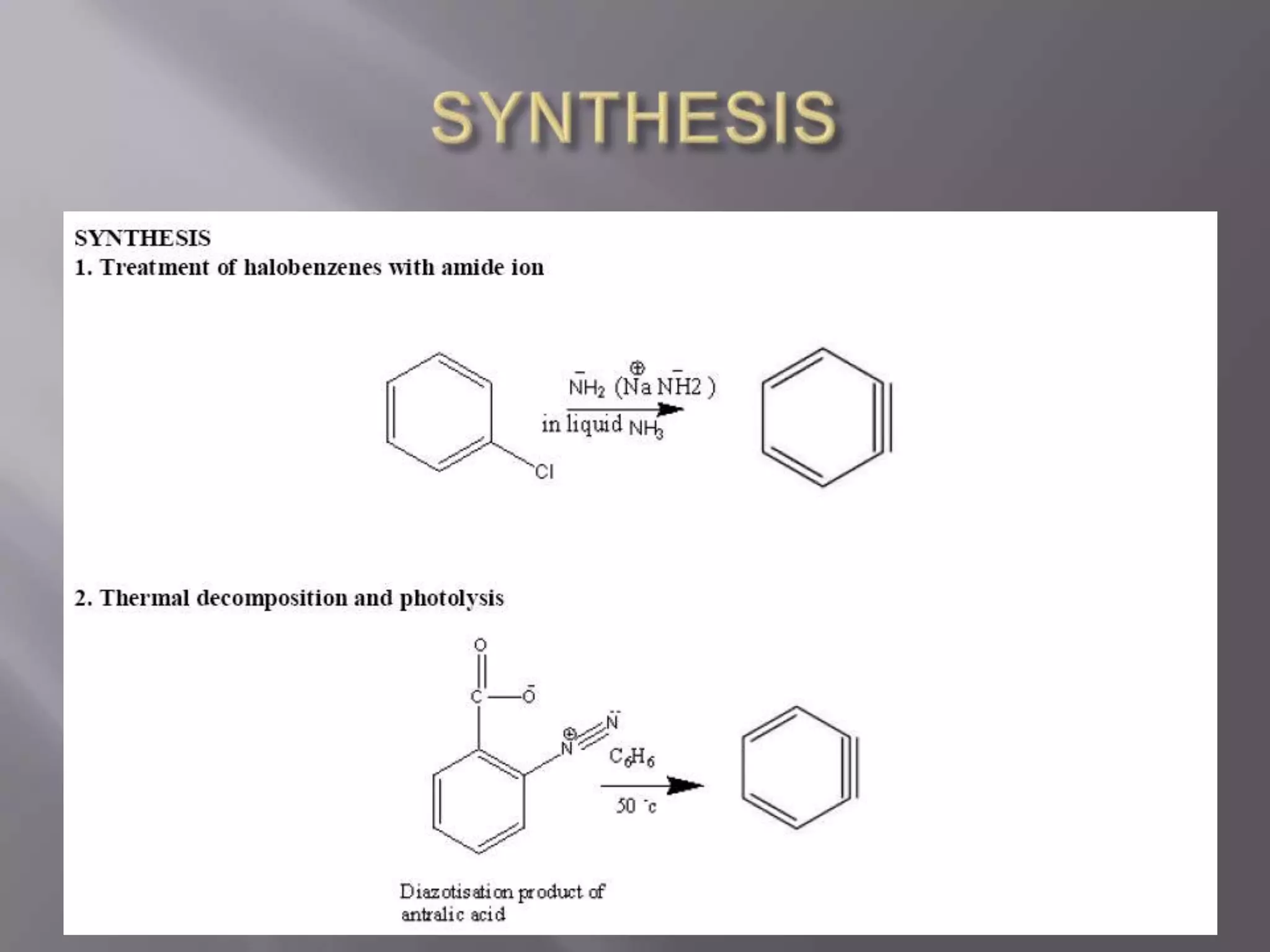
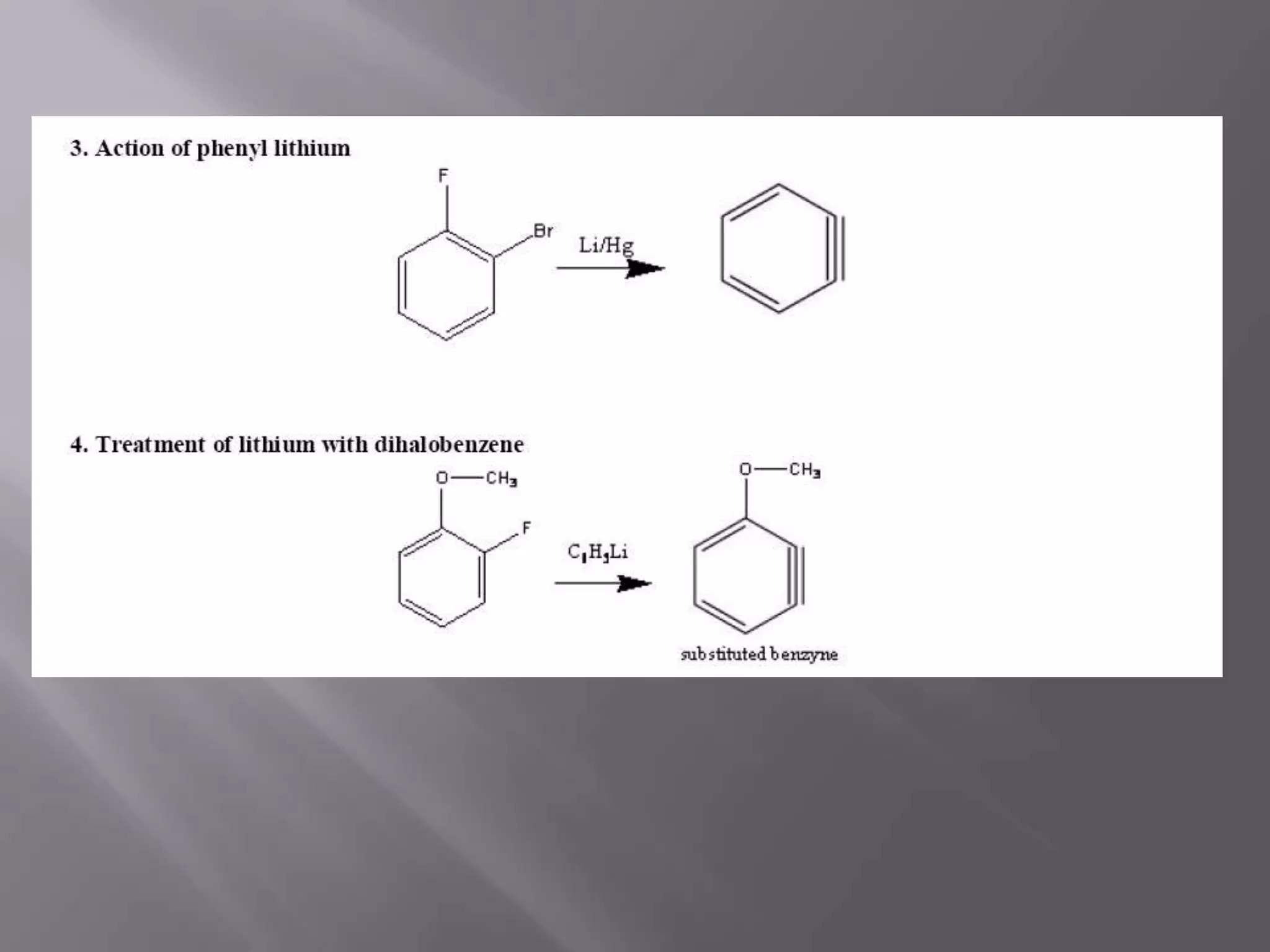
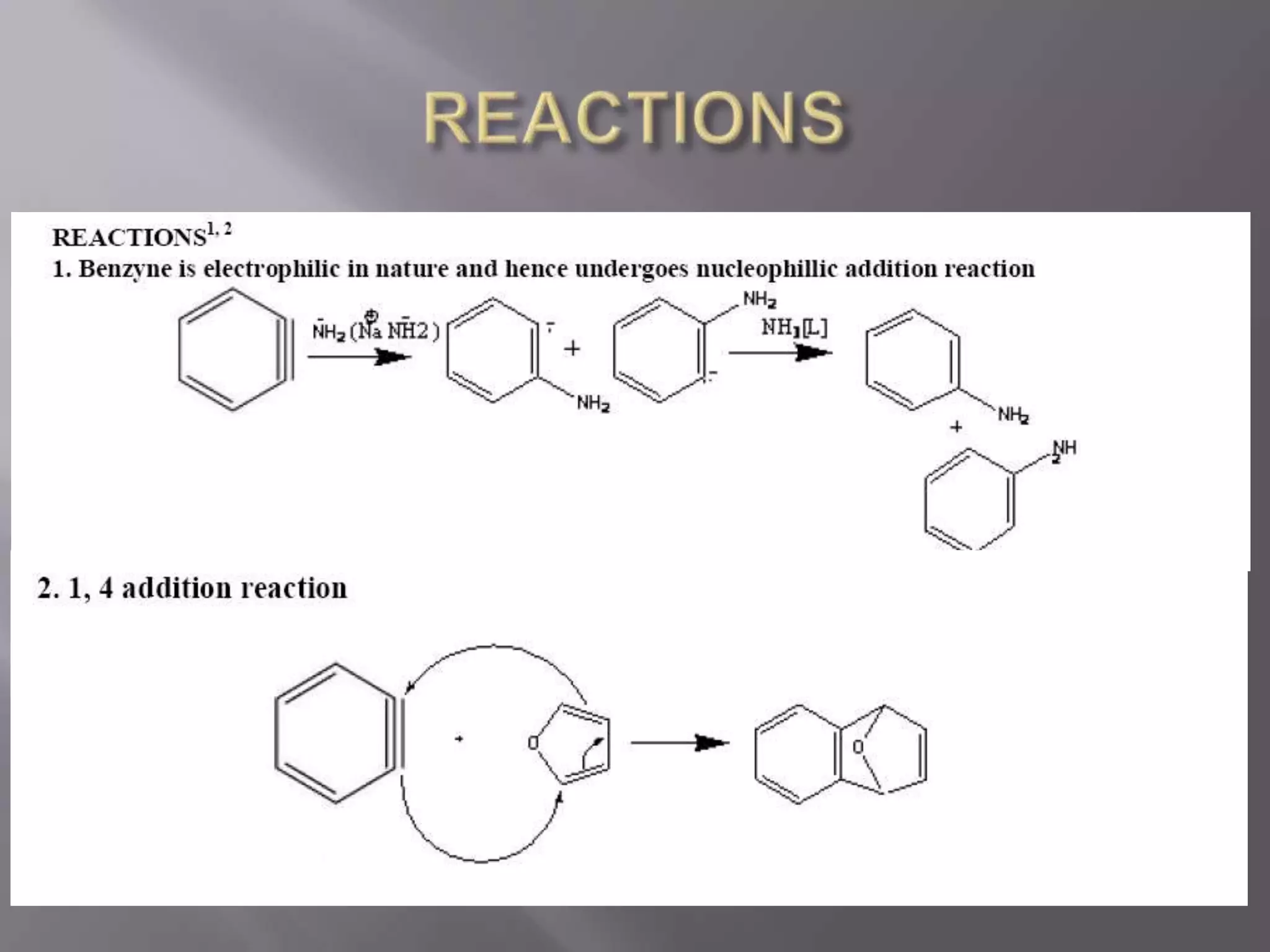
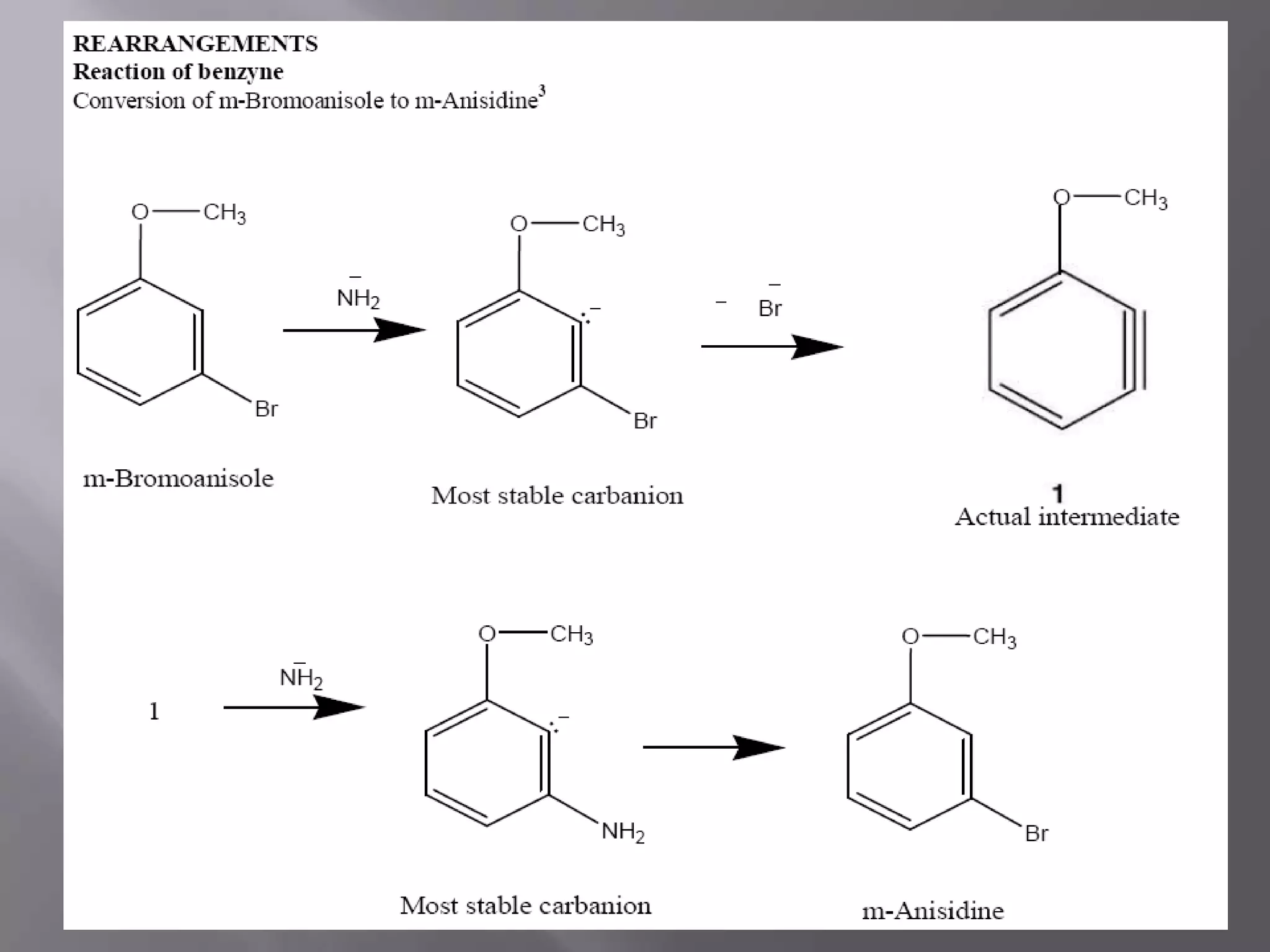
The document discusses various reactive intermediates in chemical reactions:
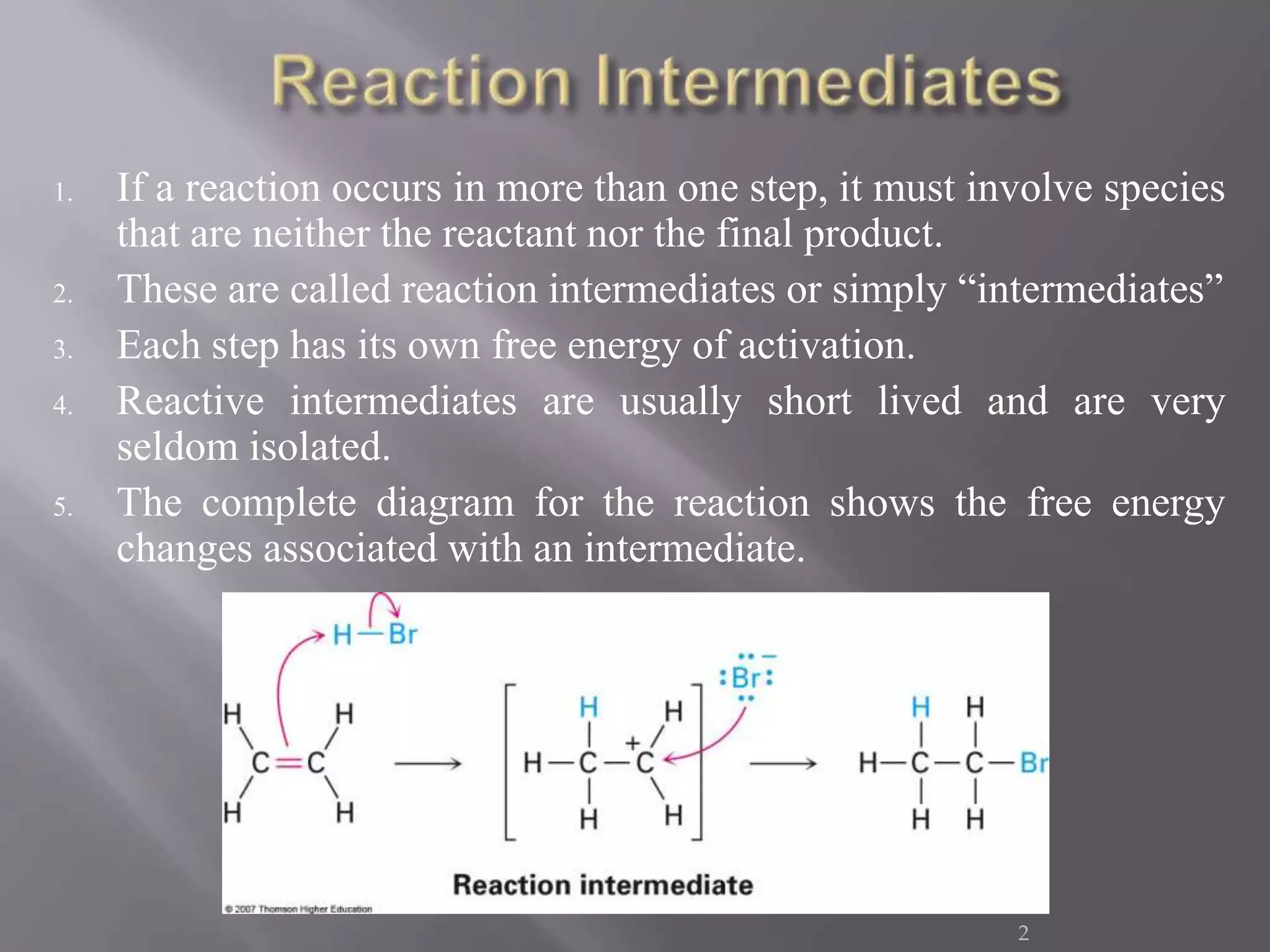
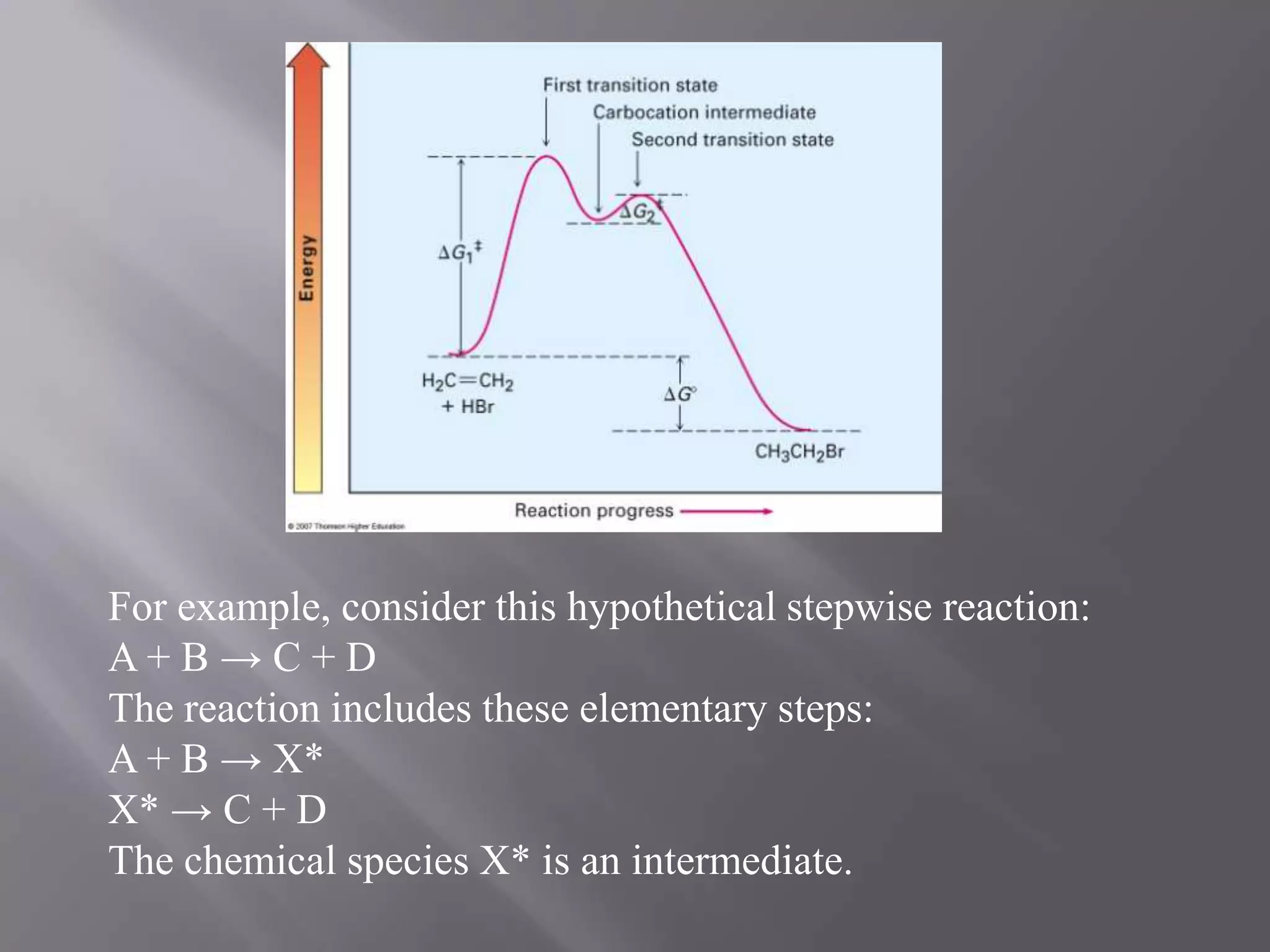
1. Intermediates are chemical species that are neither the starting reactants nor the final products but appear as transient intermediates in step-wise reactions.
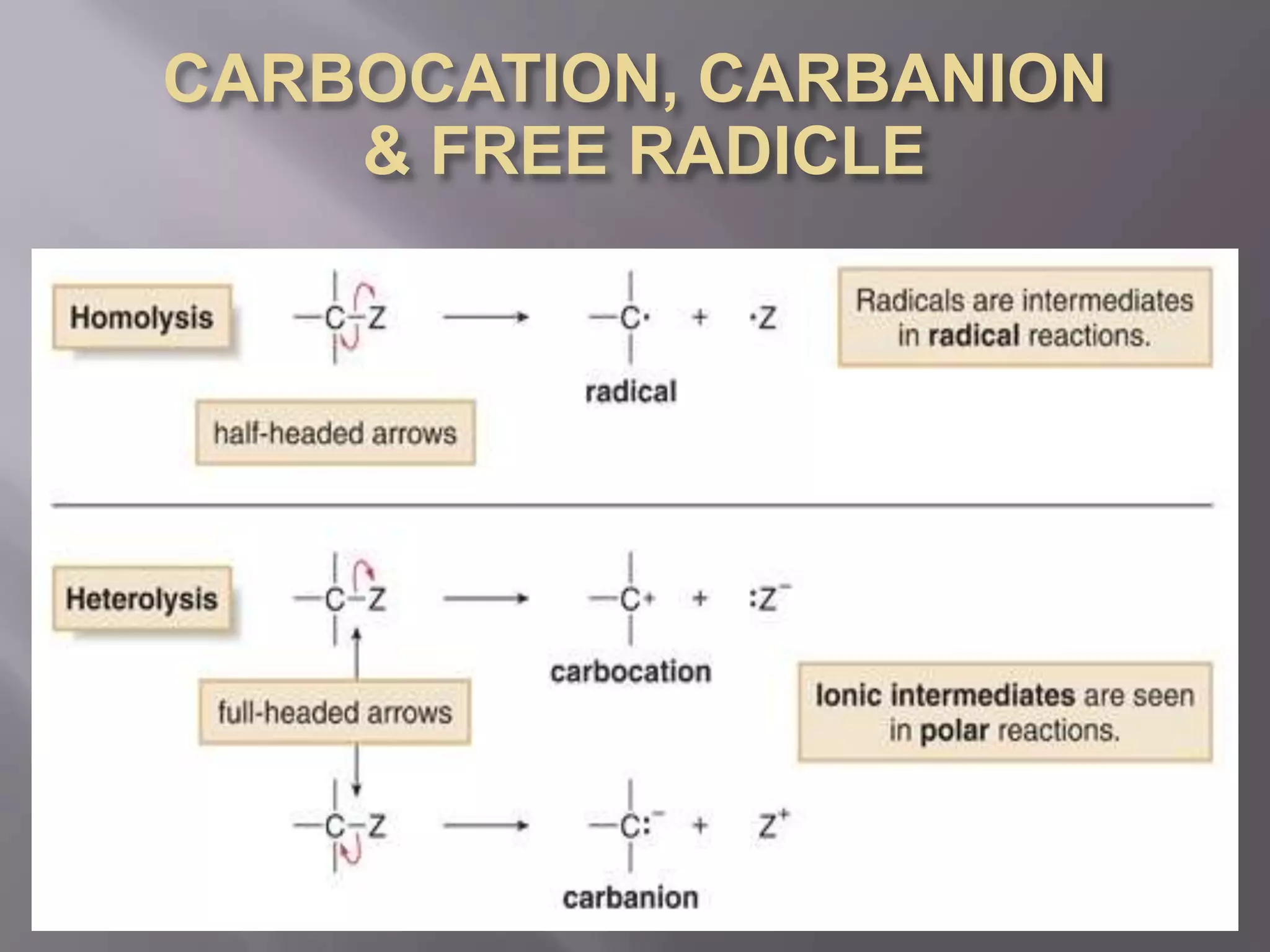
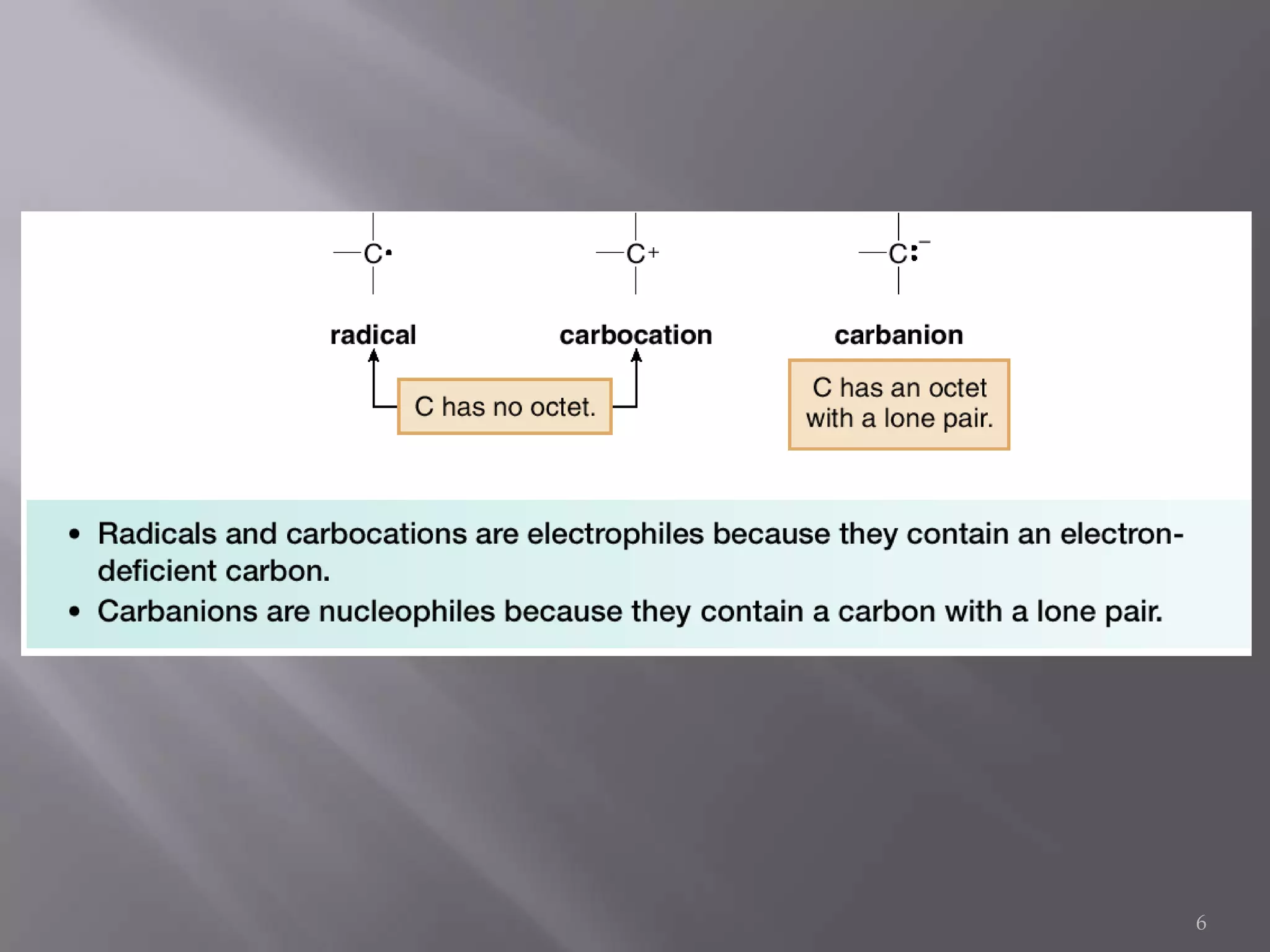
2. Common types of reactive intermediates discussed include carbocations, carbanions, free radicals, carbenes, nitrenes, and arenynes.
3. Specific details are provided about the electronic structure and reactivity of each intermediate.