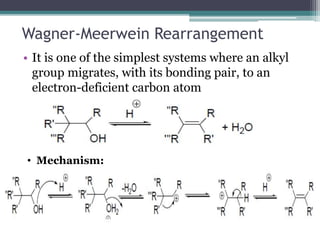
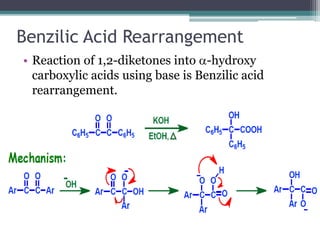
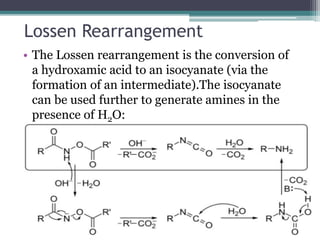
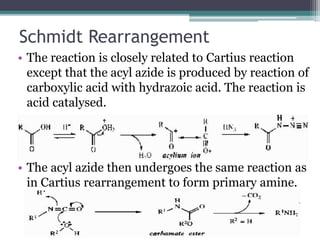
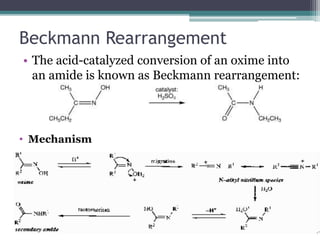
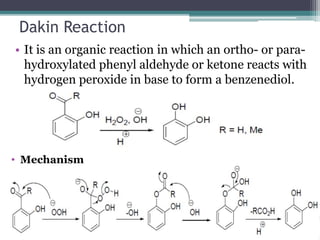
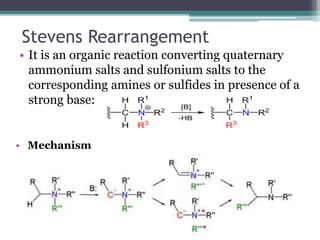
This document summarizes various types of rearrangement reactions in organic chemistry. It describes 15 categories of rearrangements including rearrangements to electron deficient carbons, nitrogens, and oxygens. For each category, 1-2 specific rearrangements are explained in more detail, including their mechanisms. Rearrangements discussed include the Wagner-Meerwein, Pinacol, Benzilic acid, Hofmann, Curtius, Lossen, Beckmann, Baeyer-Villiger, Stevens, Sommelet-Hauser, Wittig, Favorskii, Benzidine, Fries, and Claisen rearrangements. The document was prepared by a student as part of their coursework to provide an overview of









![Wittig Rearrangement
• The [1,2]-Wittig Rearrangement is the base-
promoted reaction of ethers to yield secondary
or tertiary alcohol:
• Mechanism](https://image.slidesharecdn.com/rearrangementreactions-chem-200506165024/85/Rearrangement-reactions-18-320.jpg)

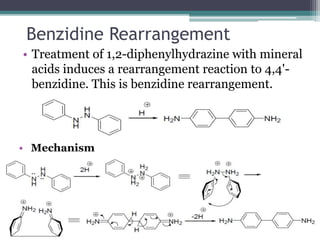
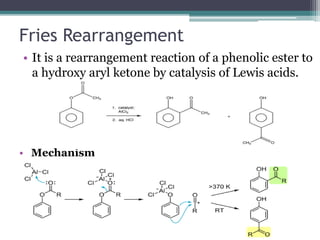
![Claisen Rearrangement
• It is a [3,3]-sigmatropic reaction where an Aryl
allyl ethers on being heating converts into
allylphenols.
• Mechanism](https://image.slidesharecdn.com/rearrangementreactions-chem-200506165024/85/Rearrangement-reactions-22-320.jpg)
