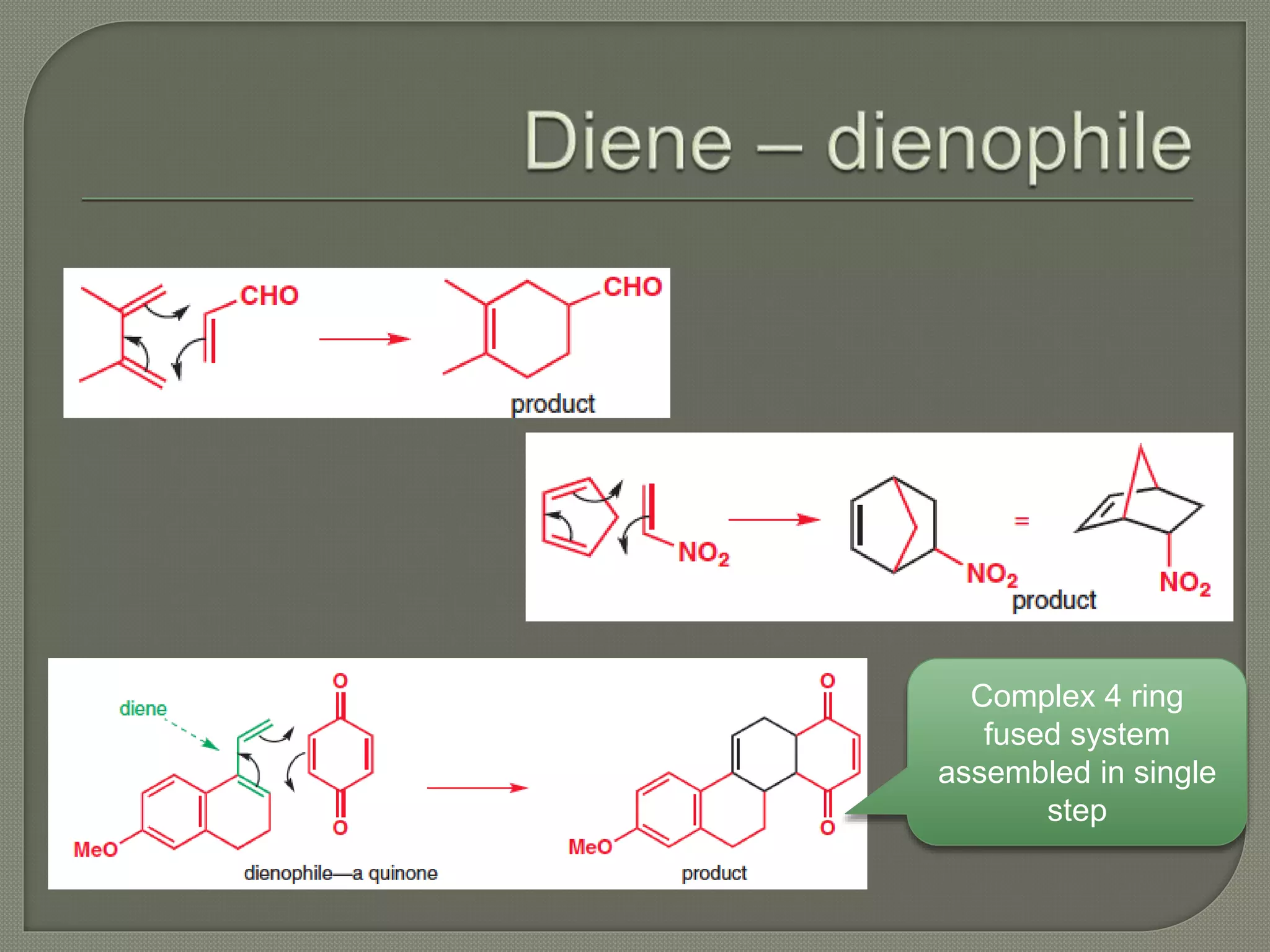
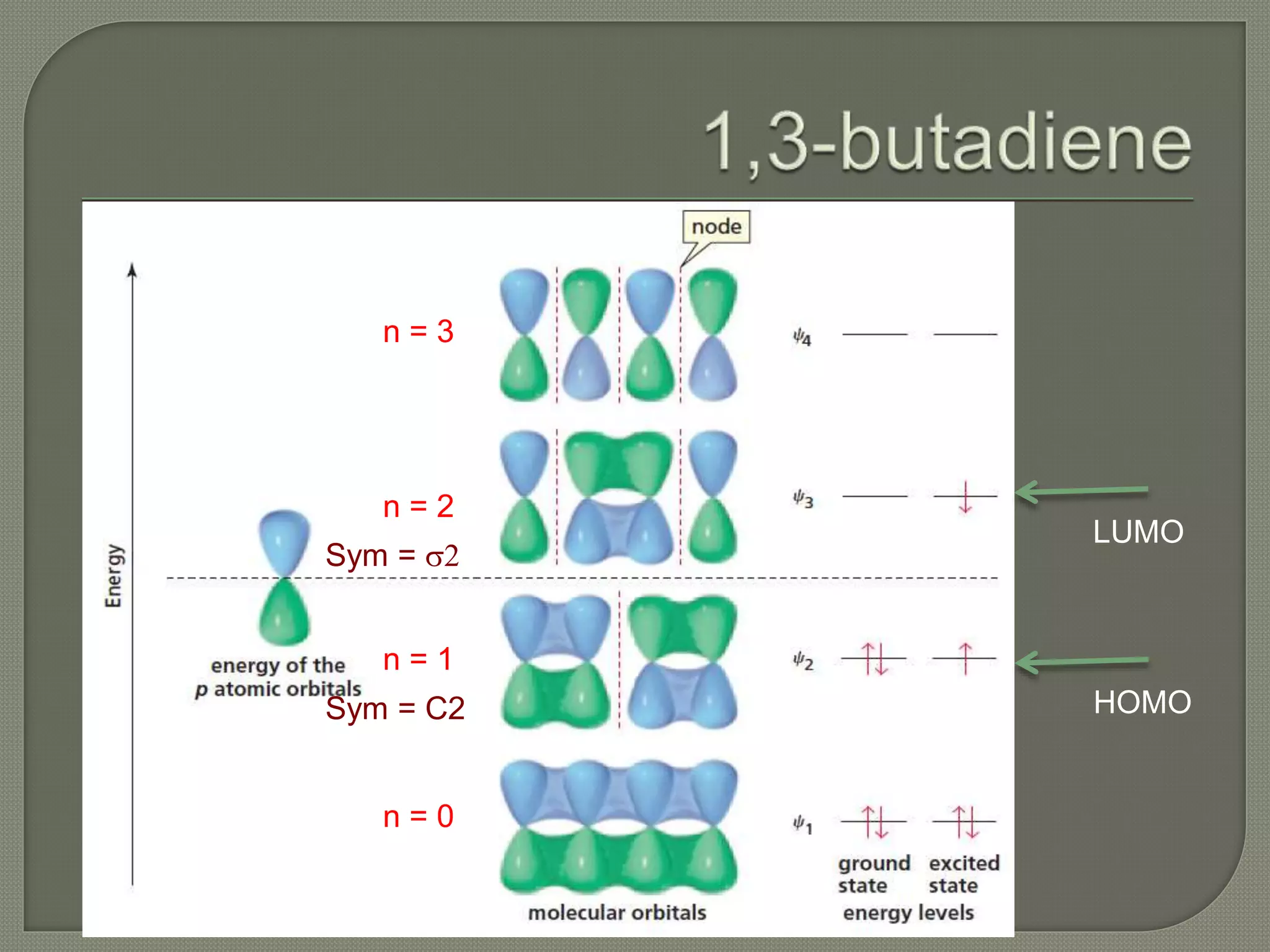
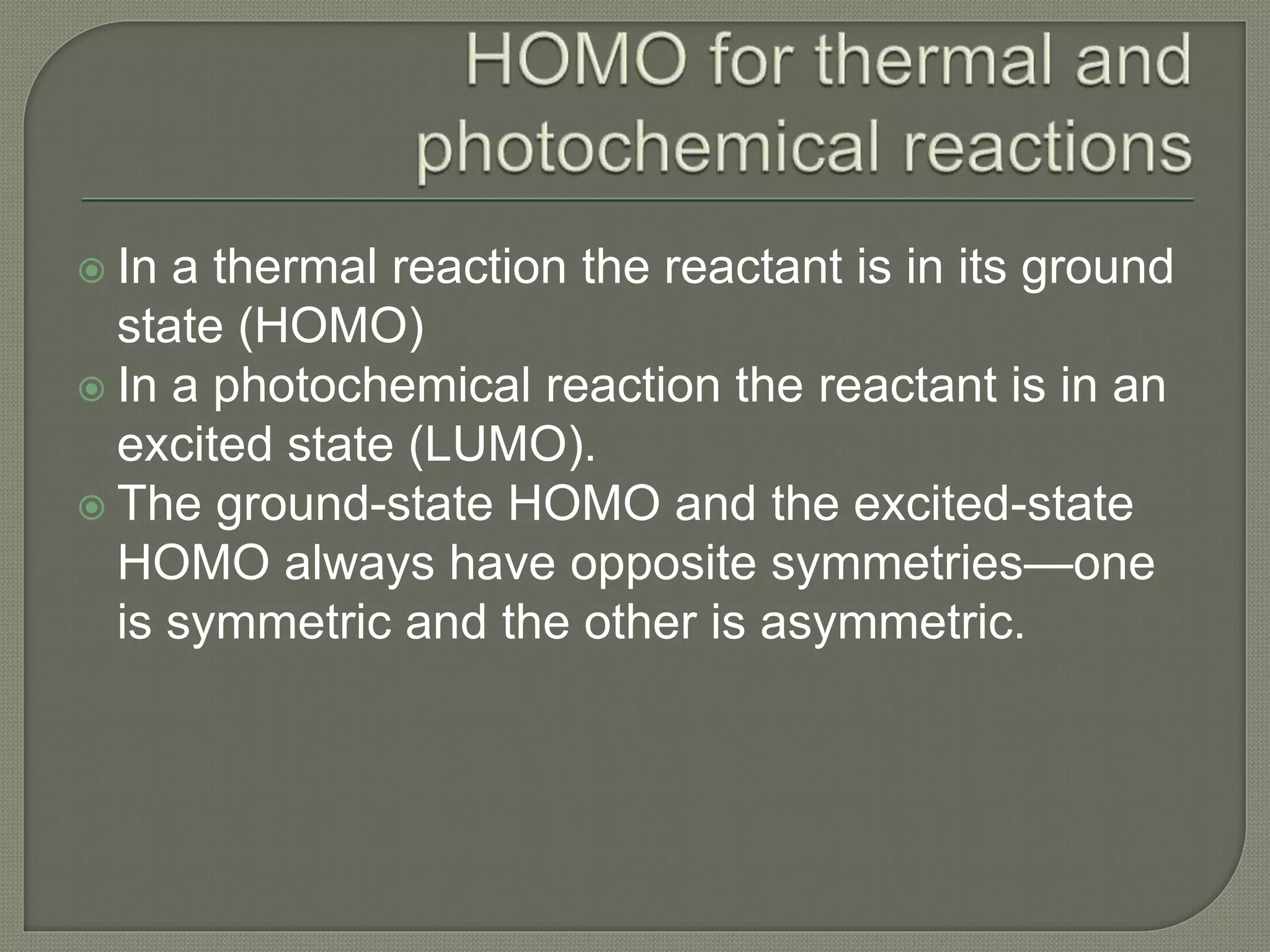
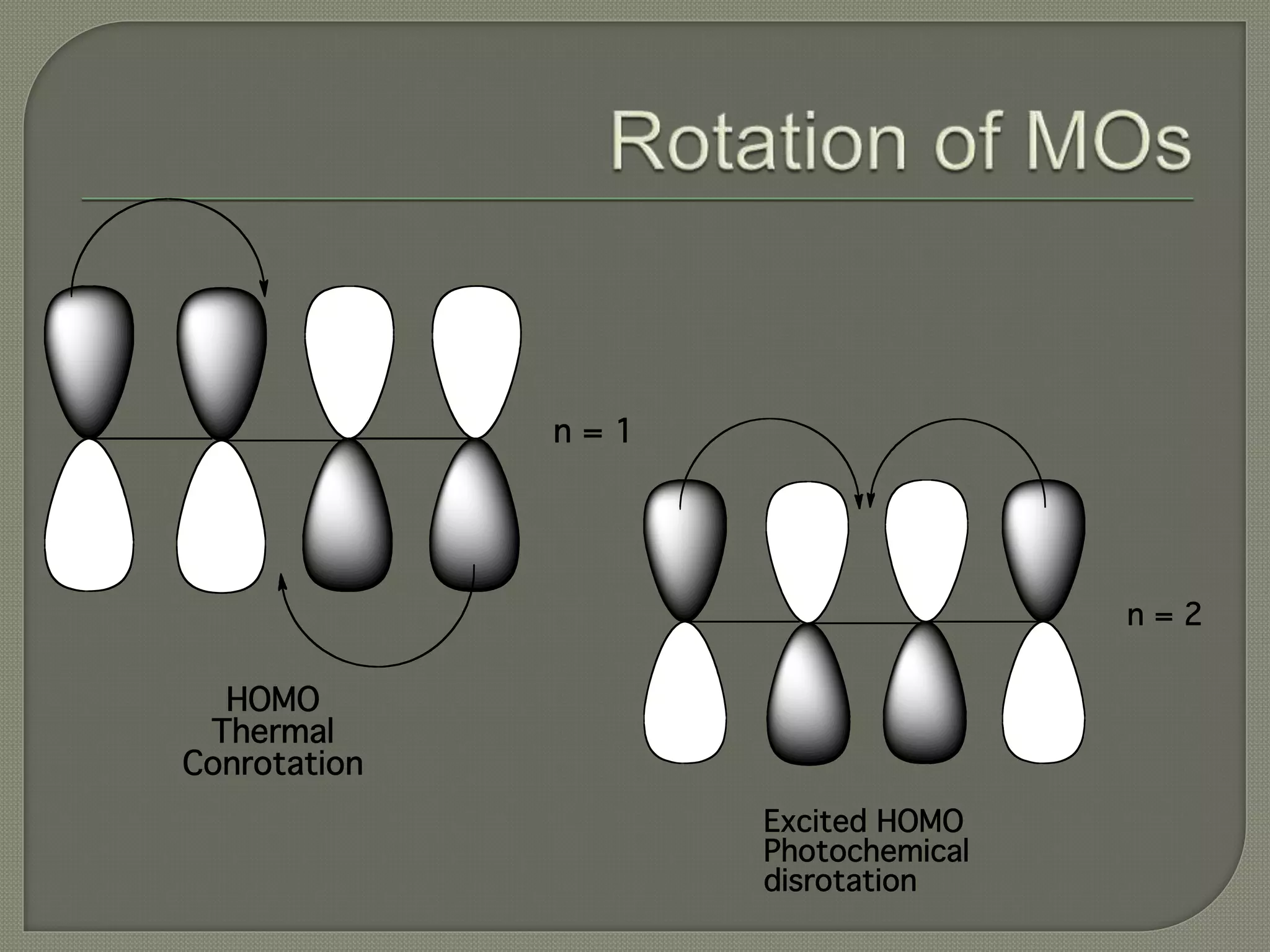
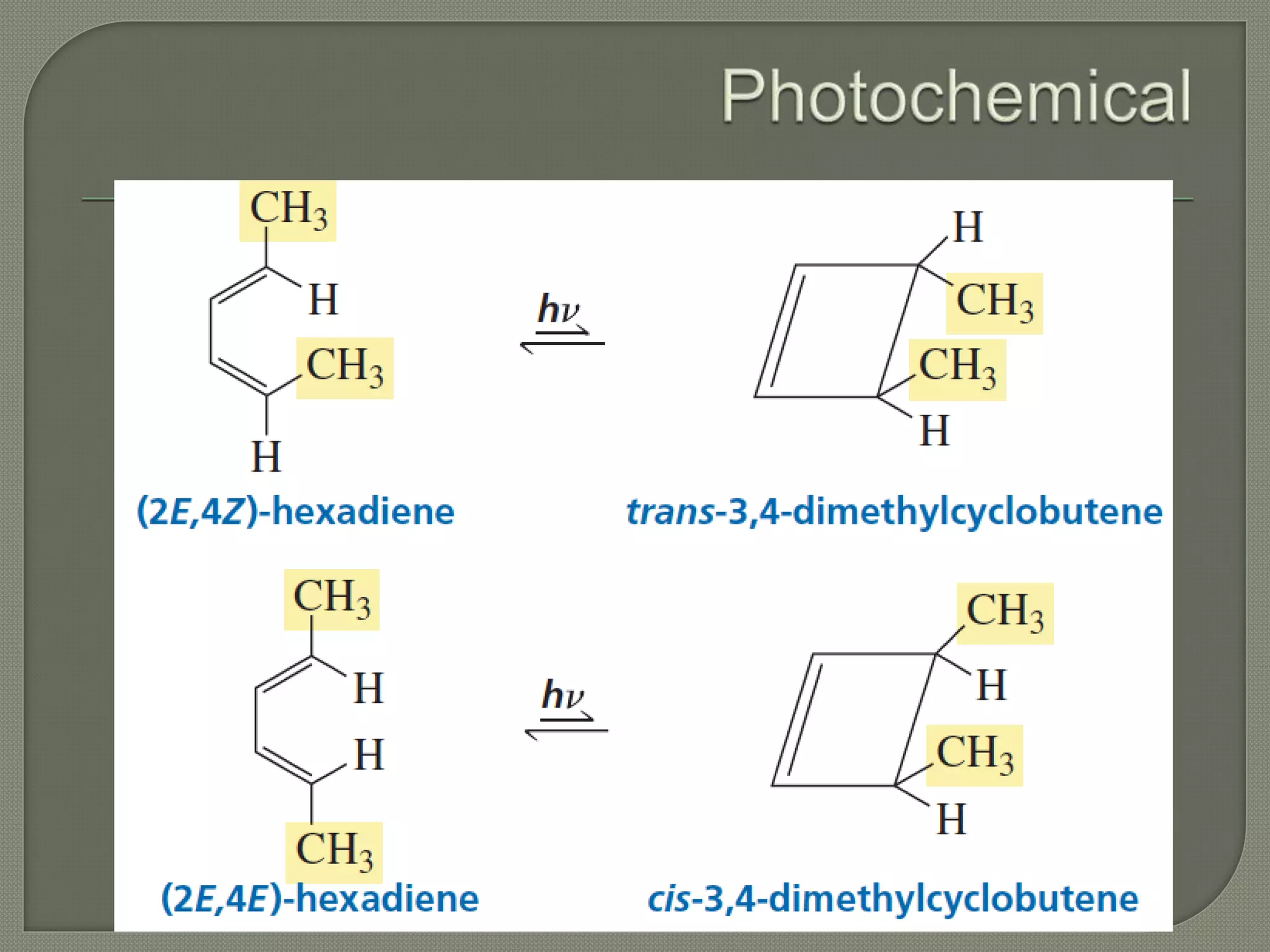
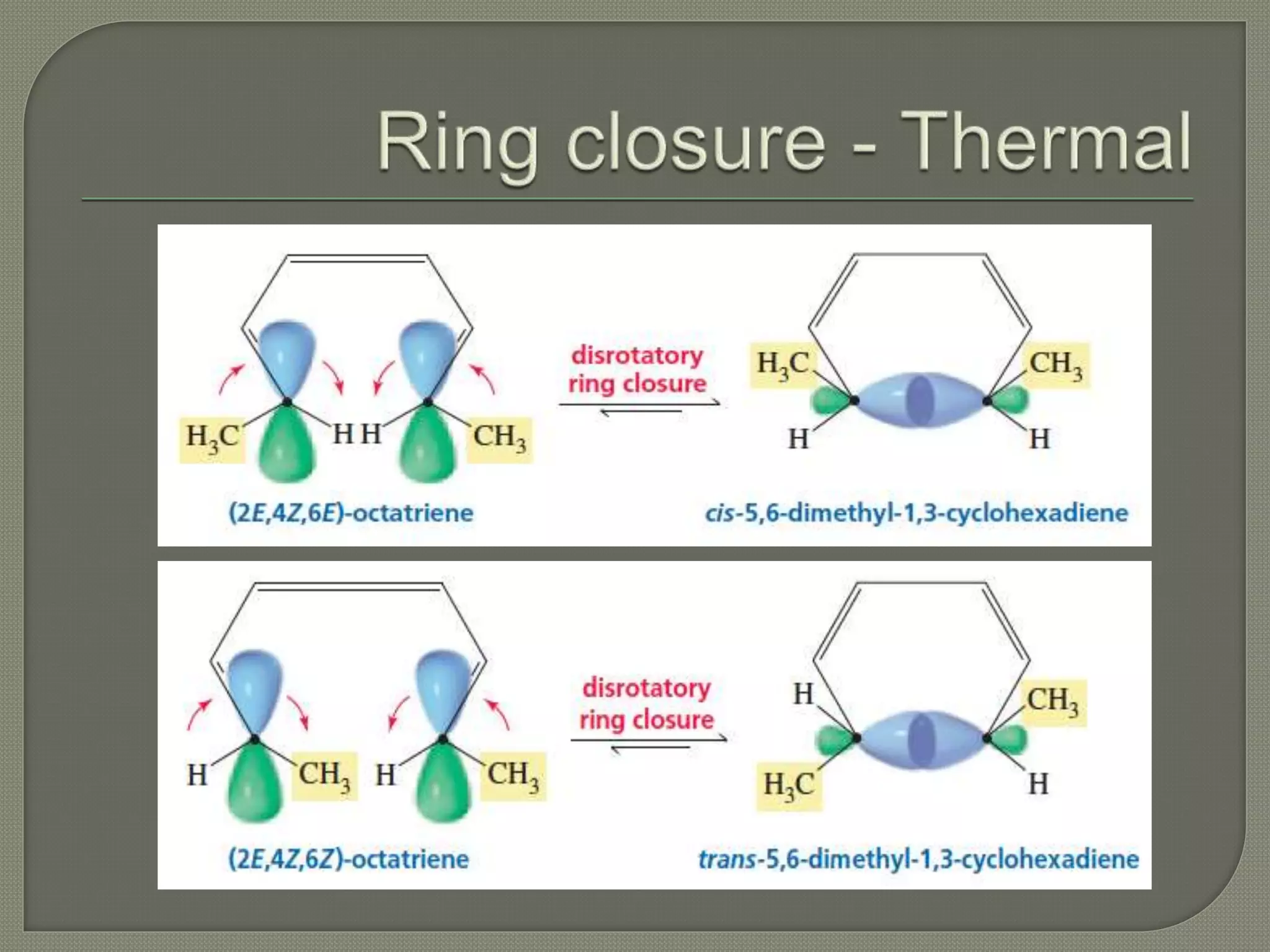
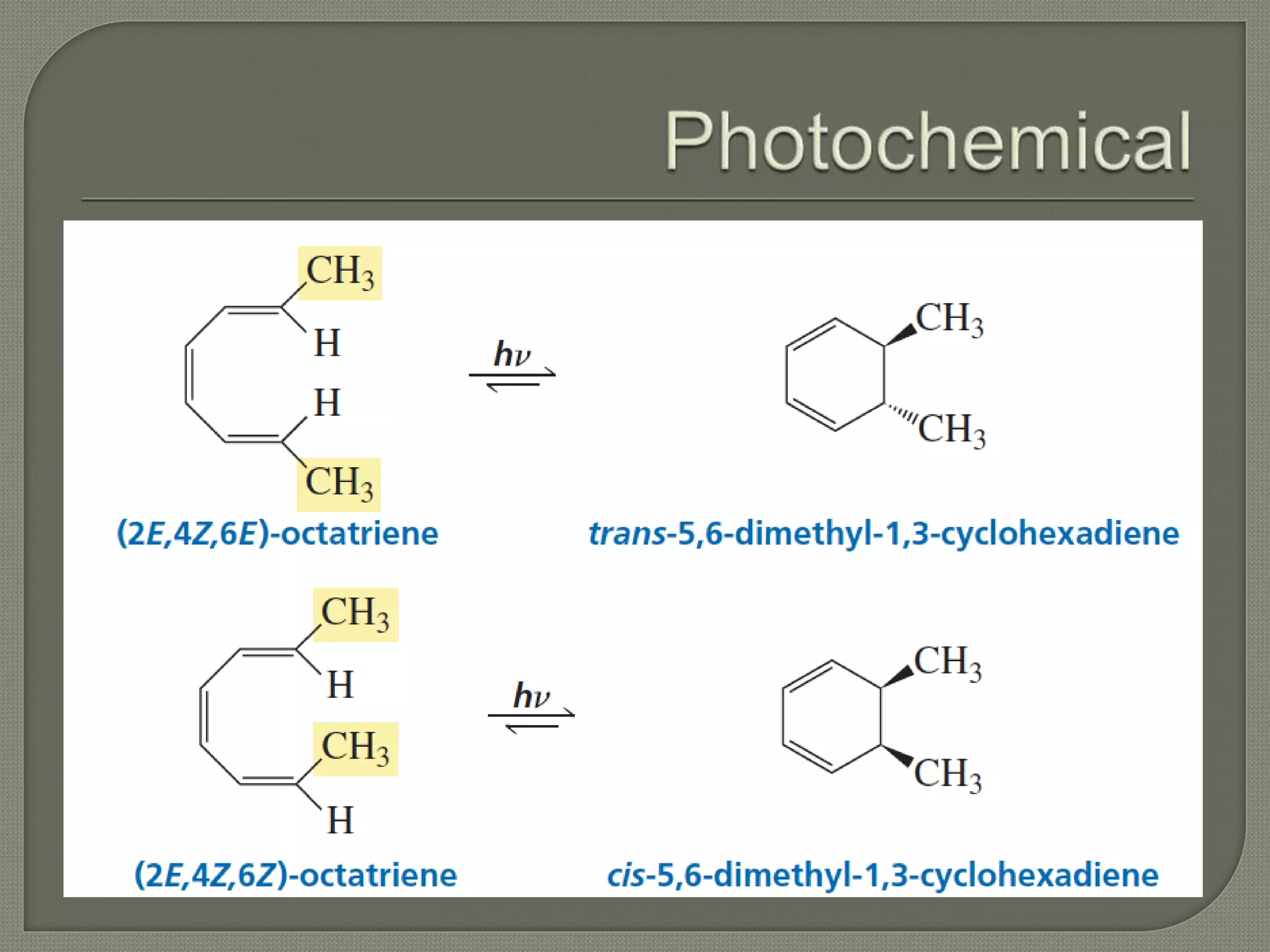
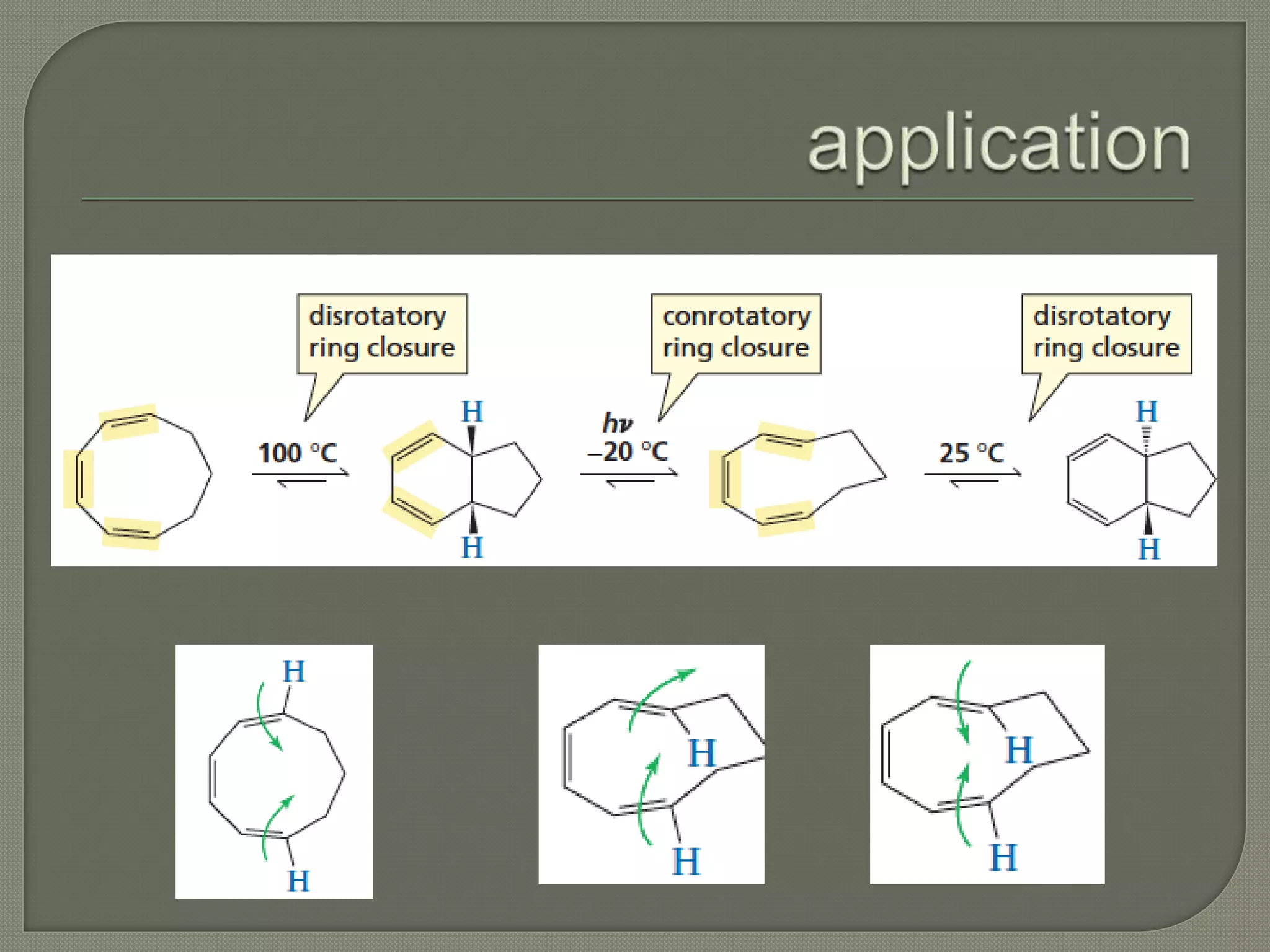
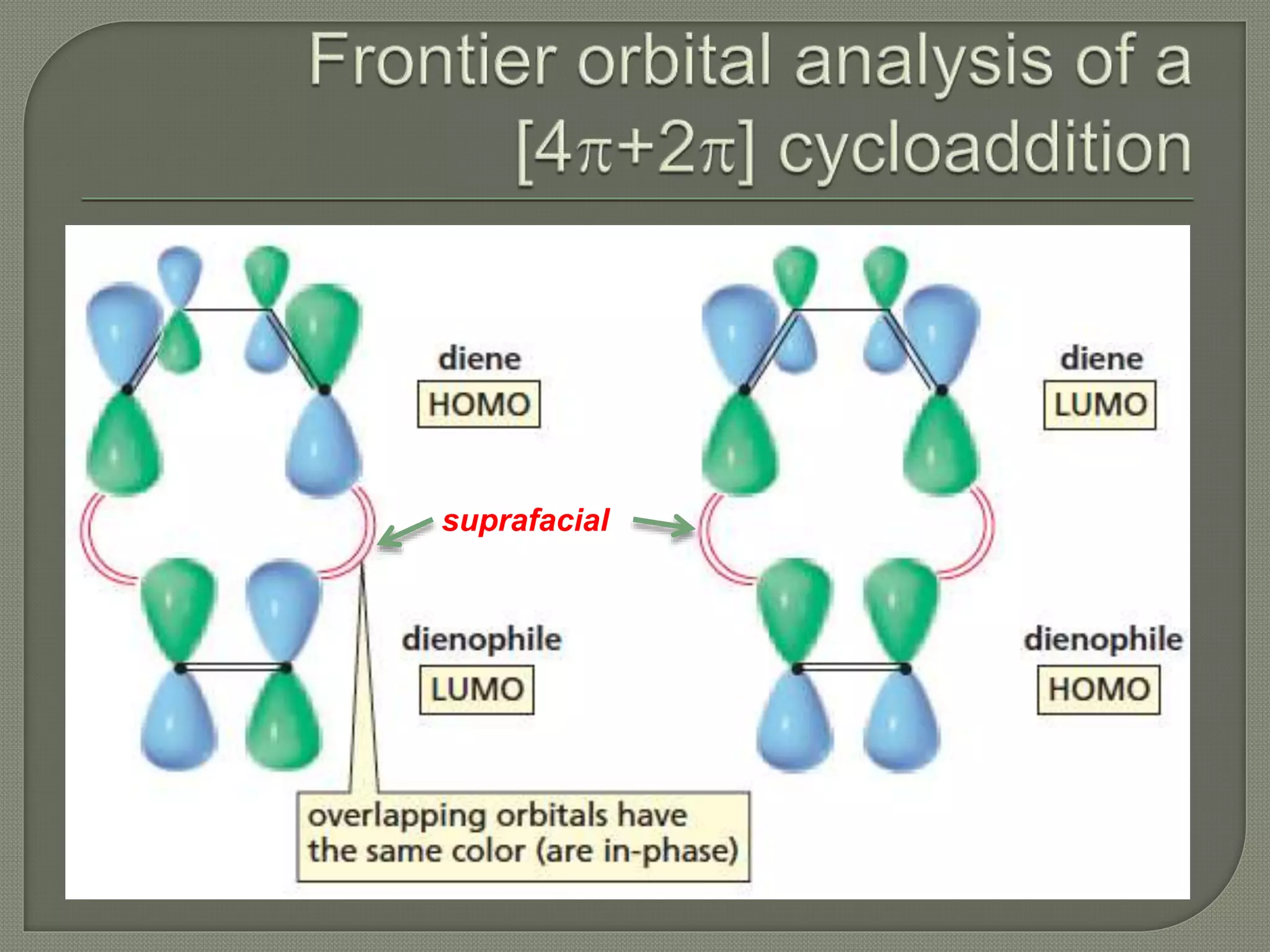
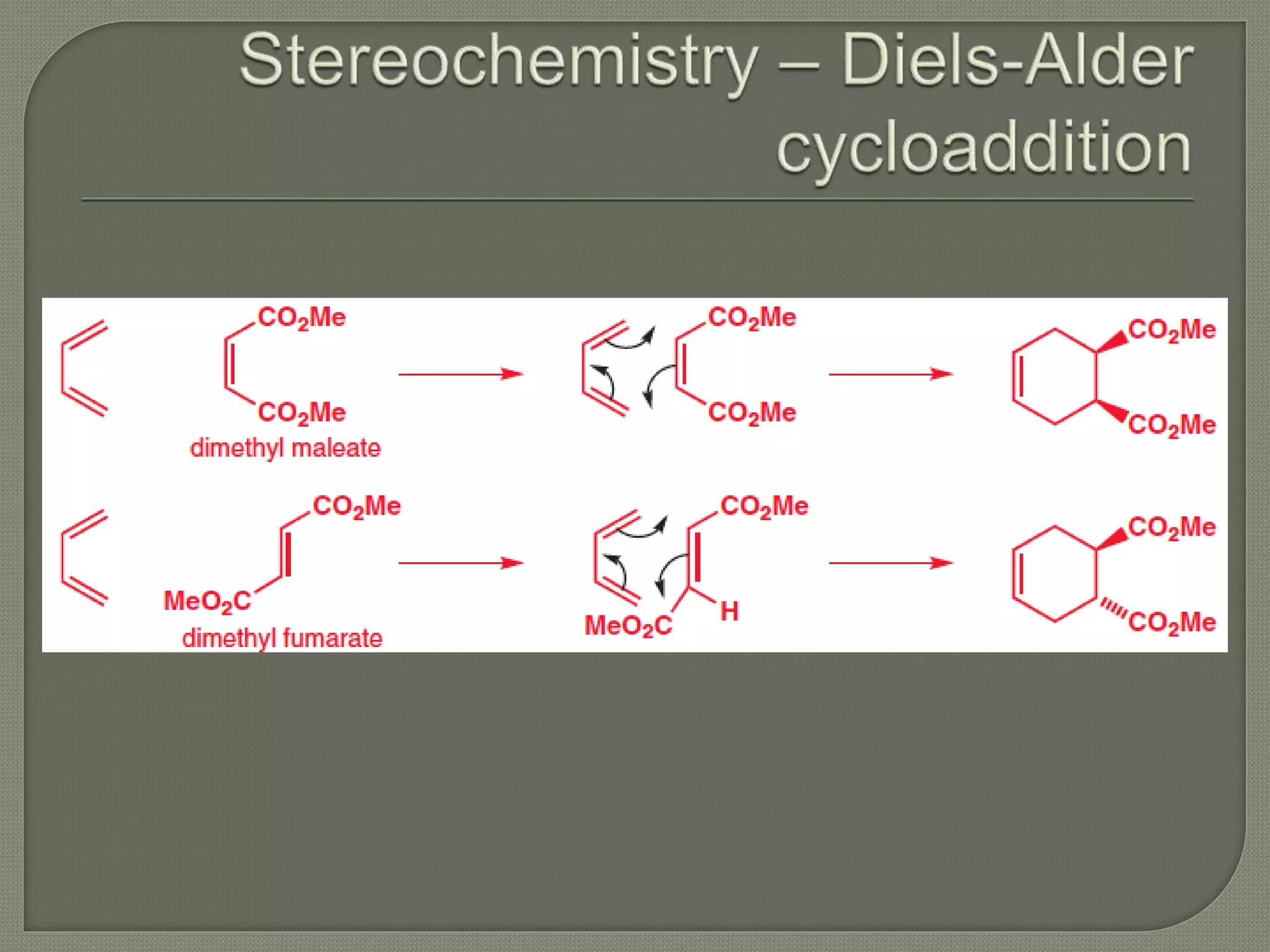
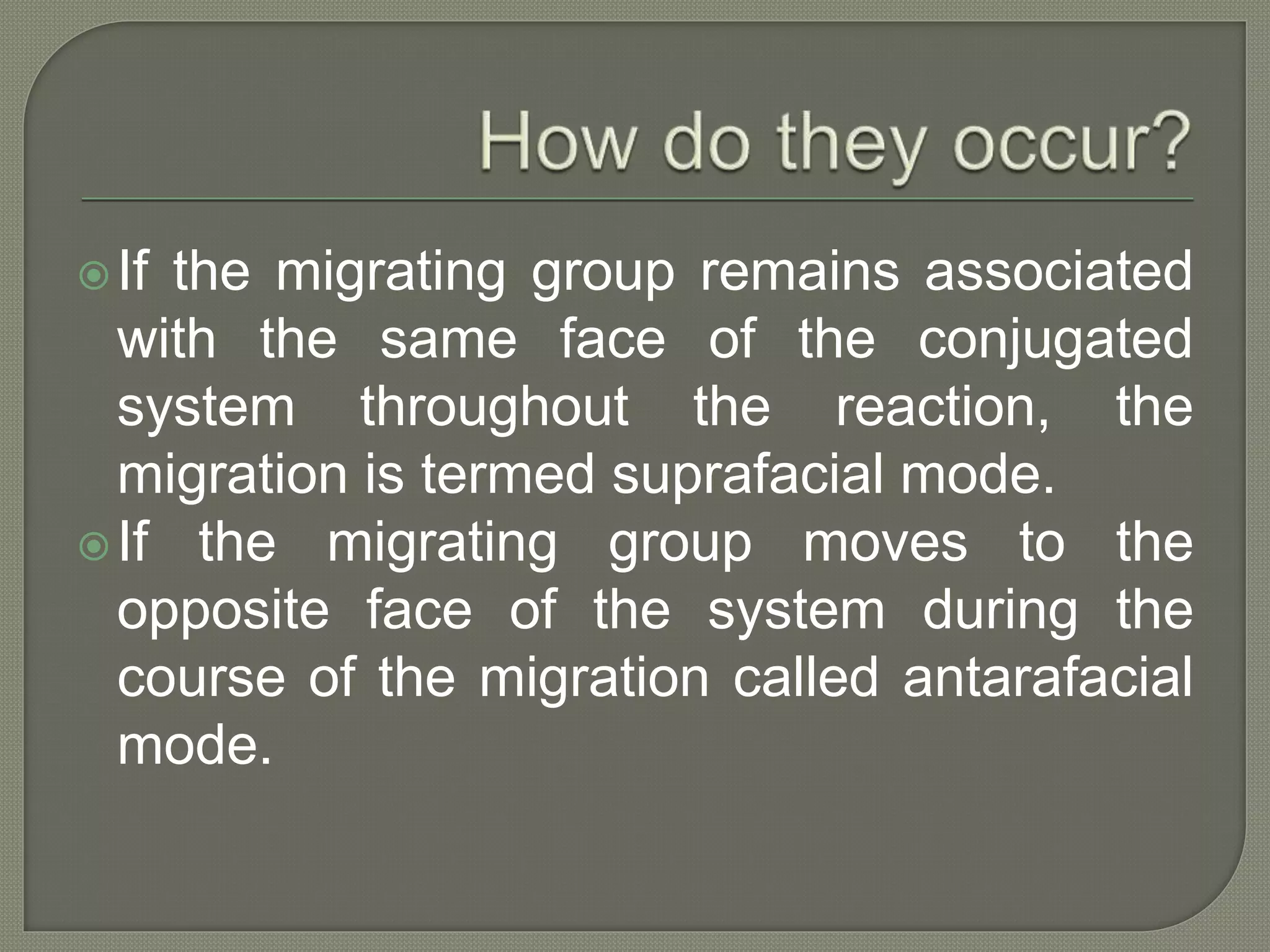
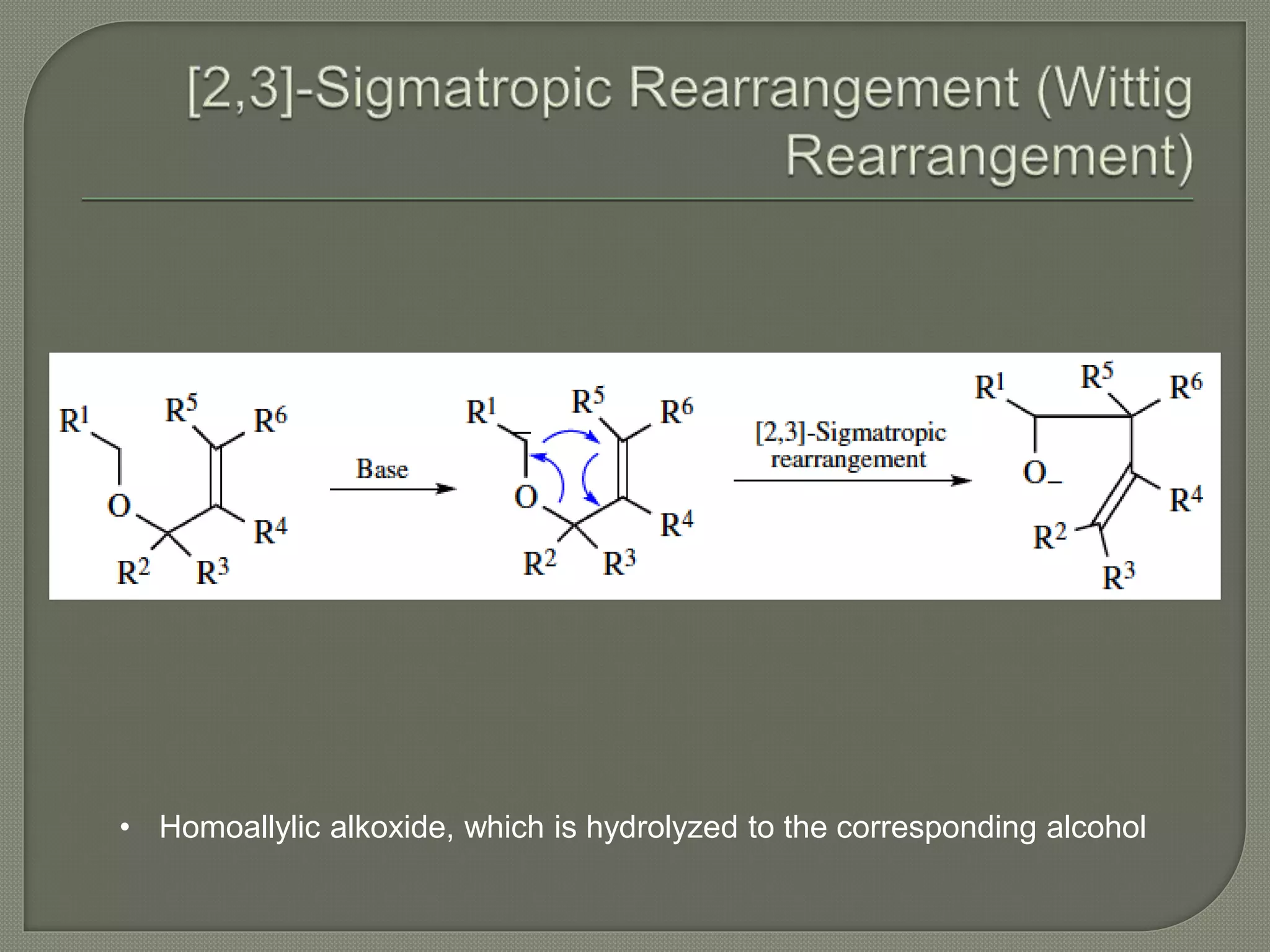
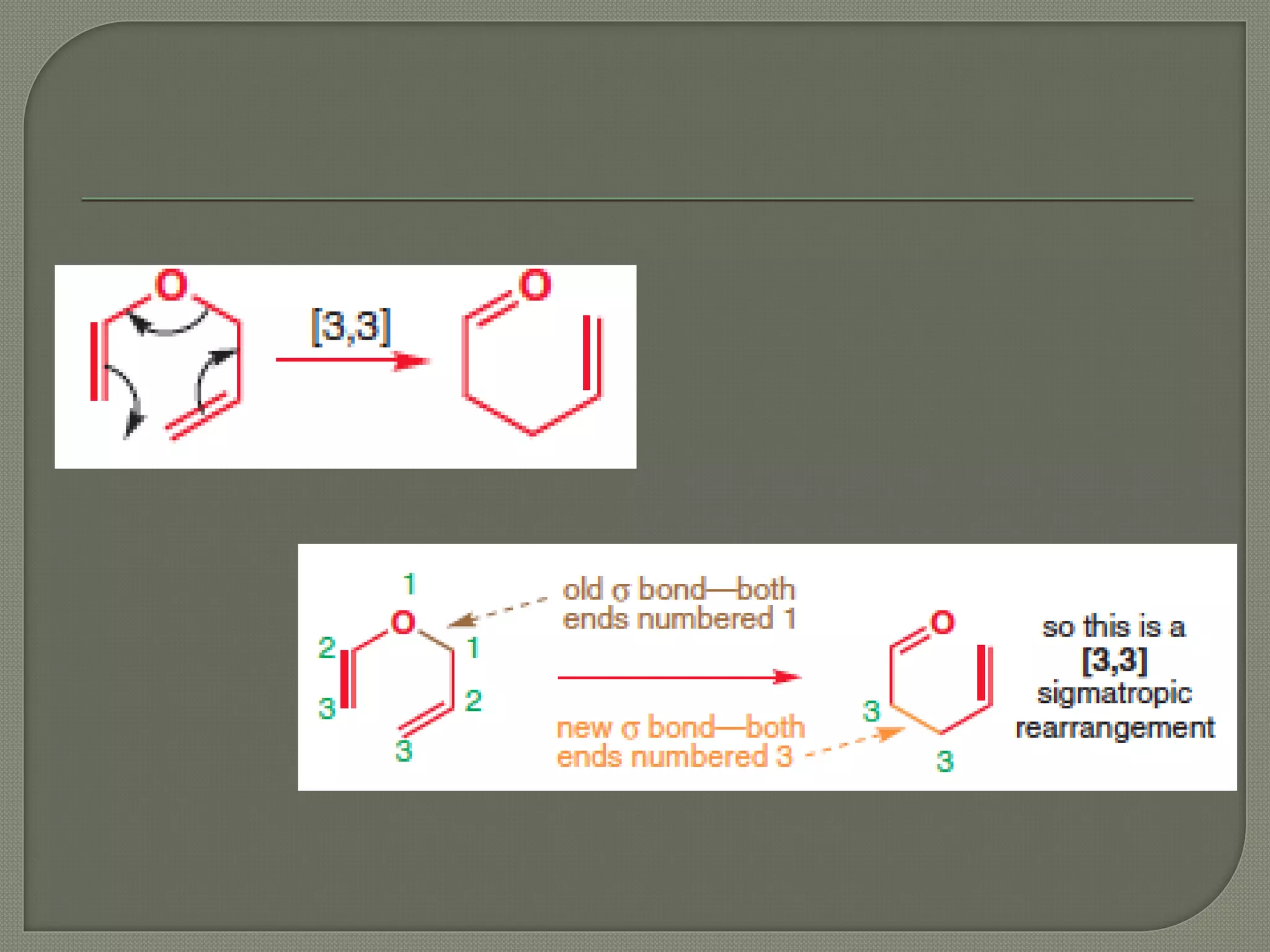
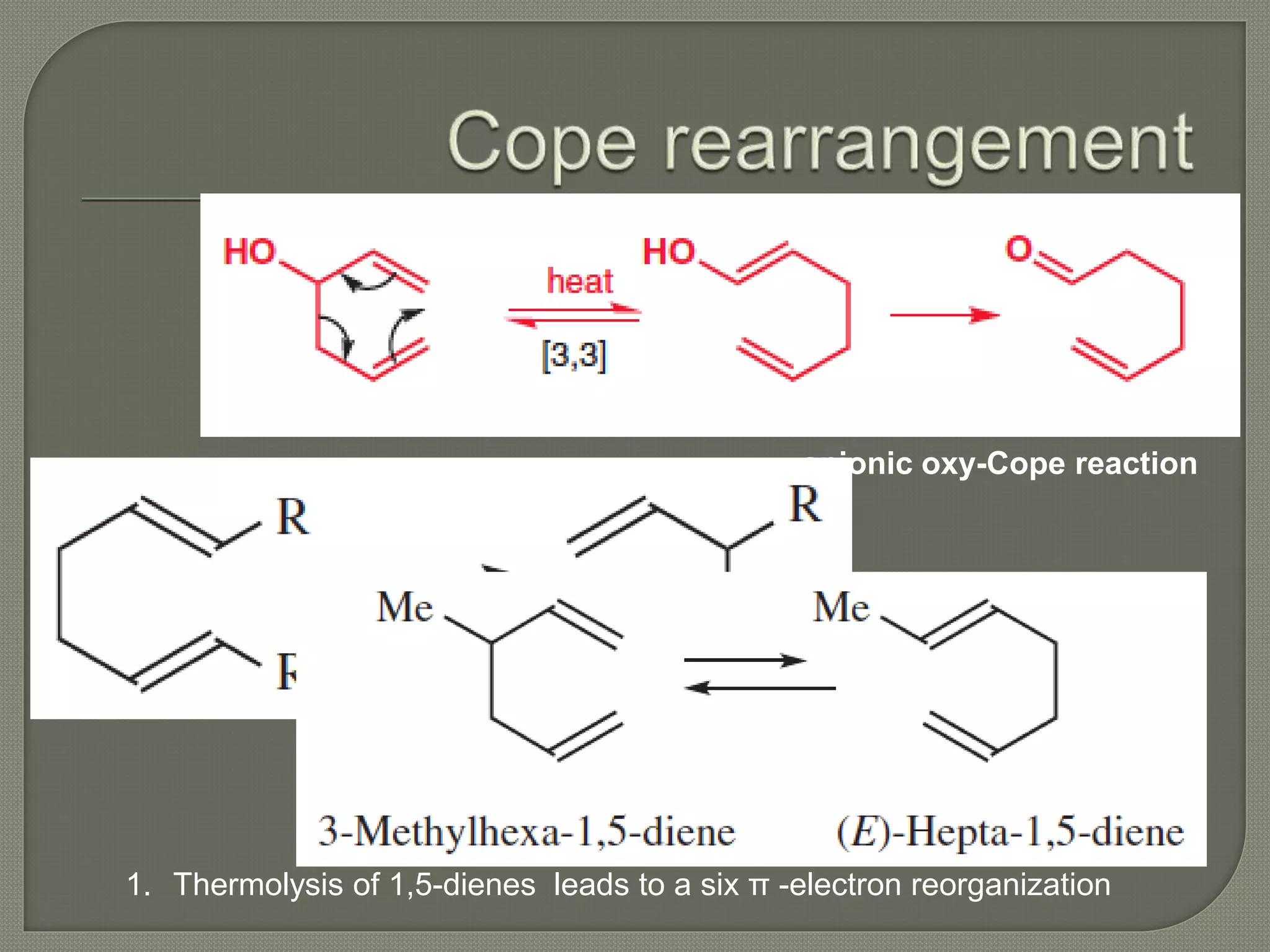
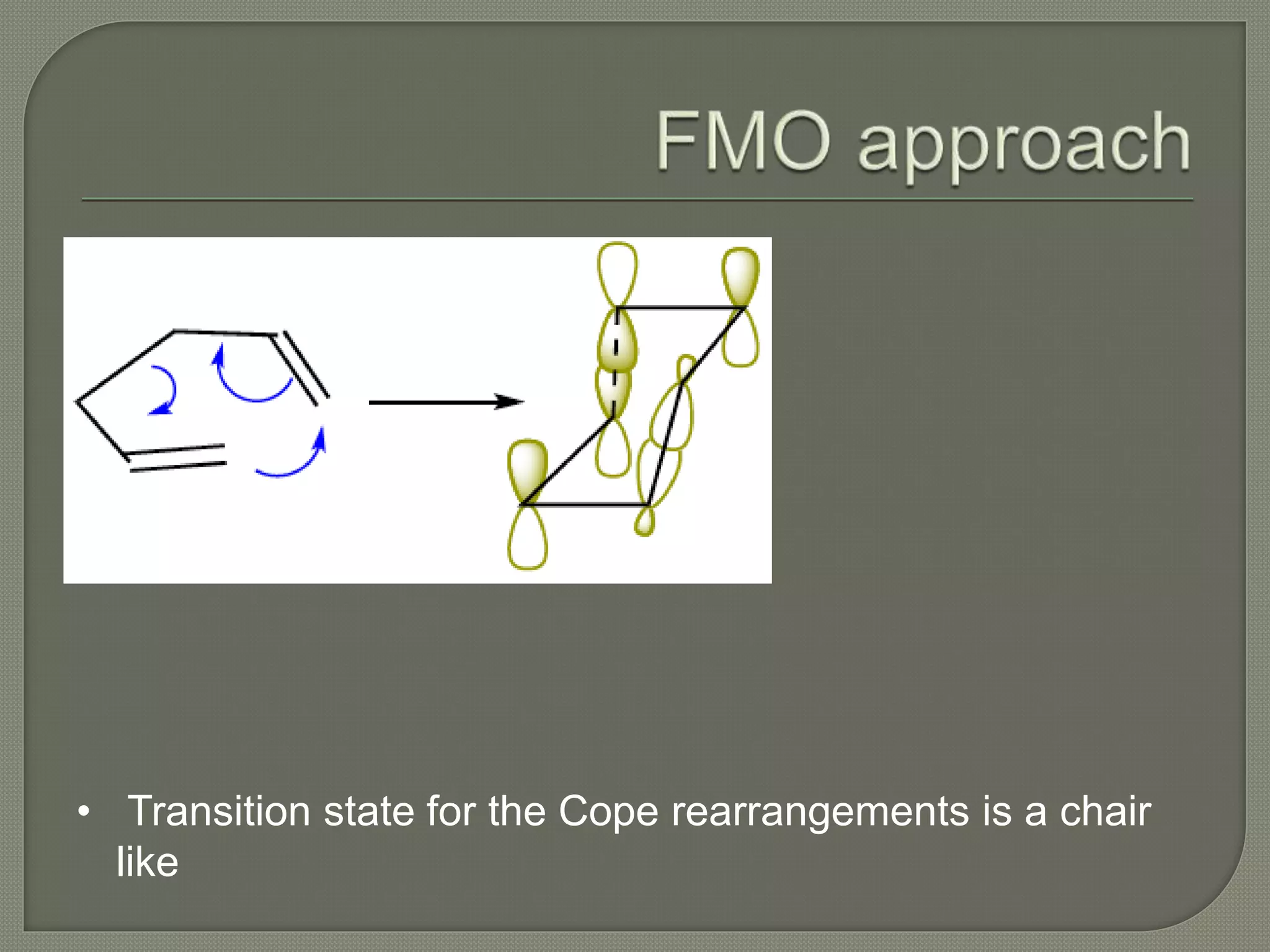
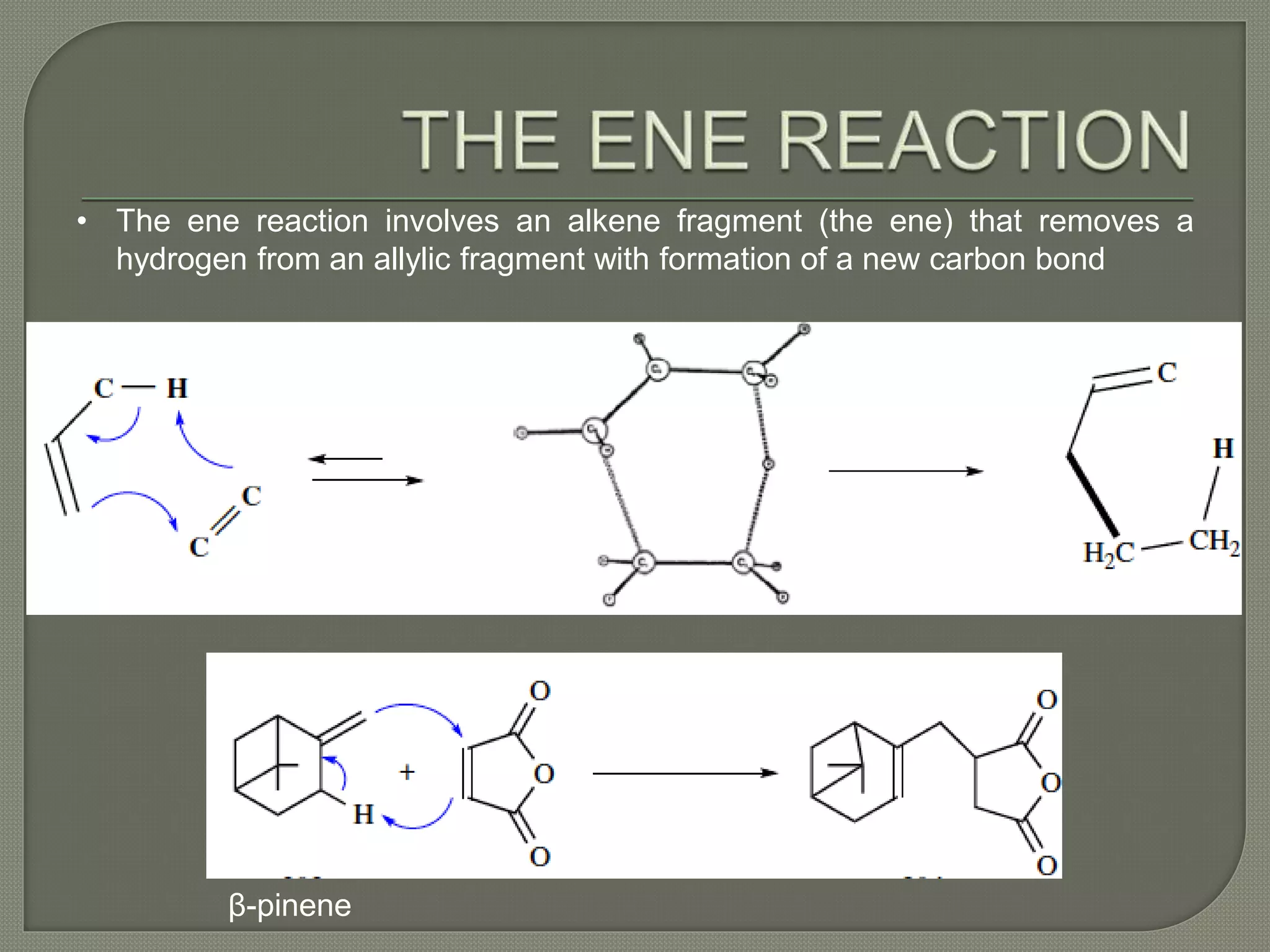
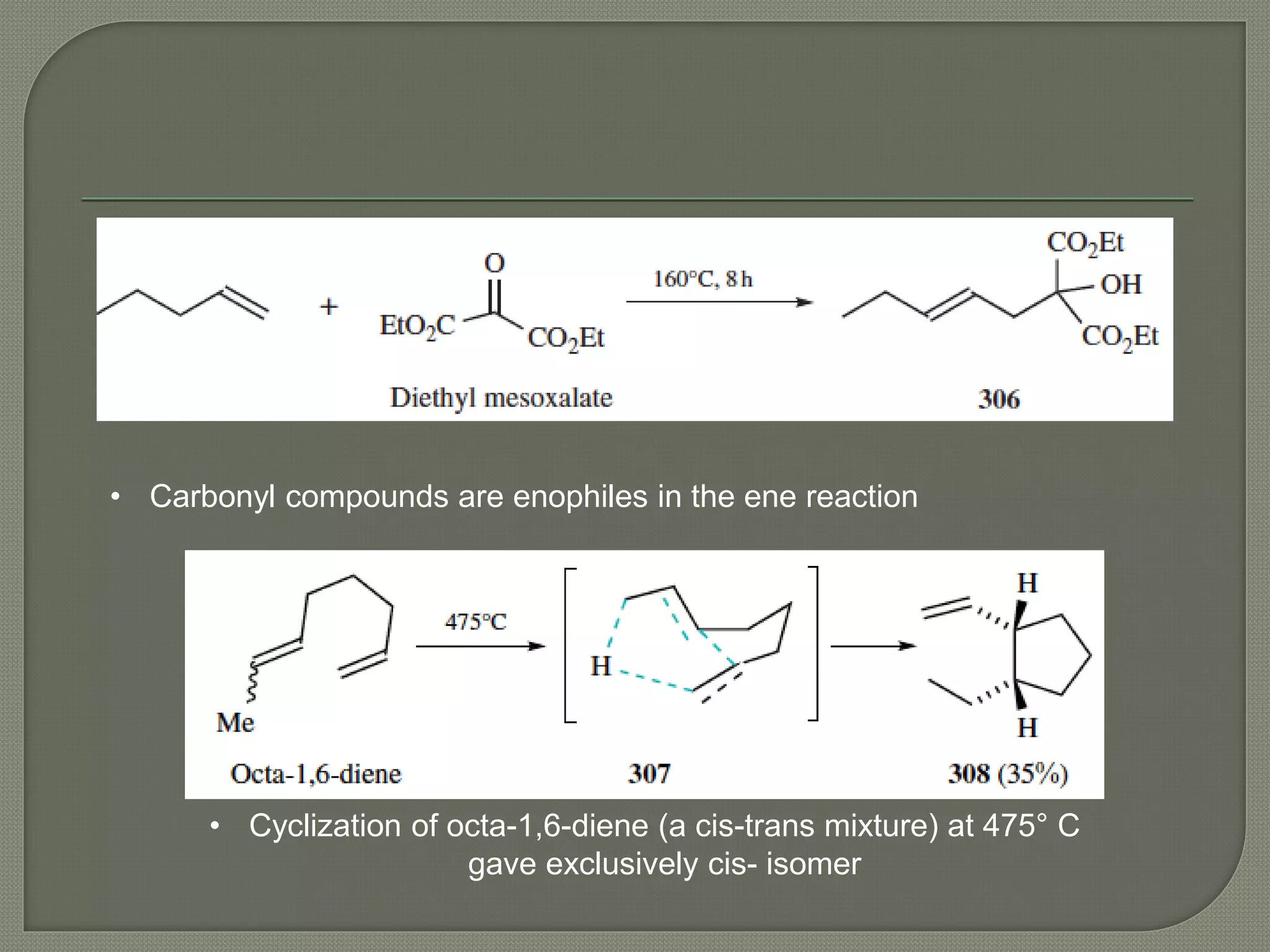
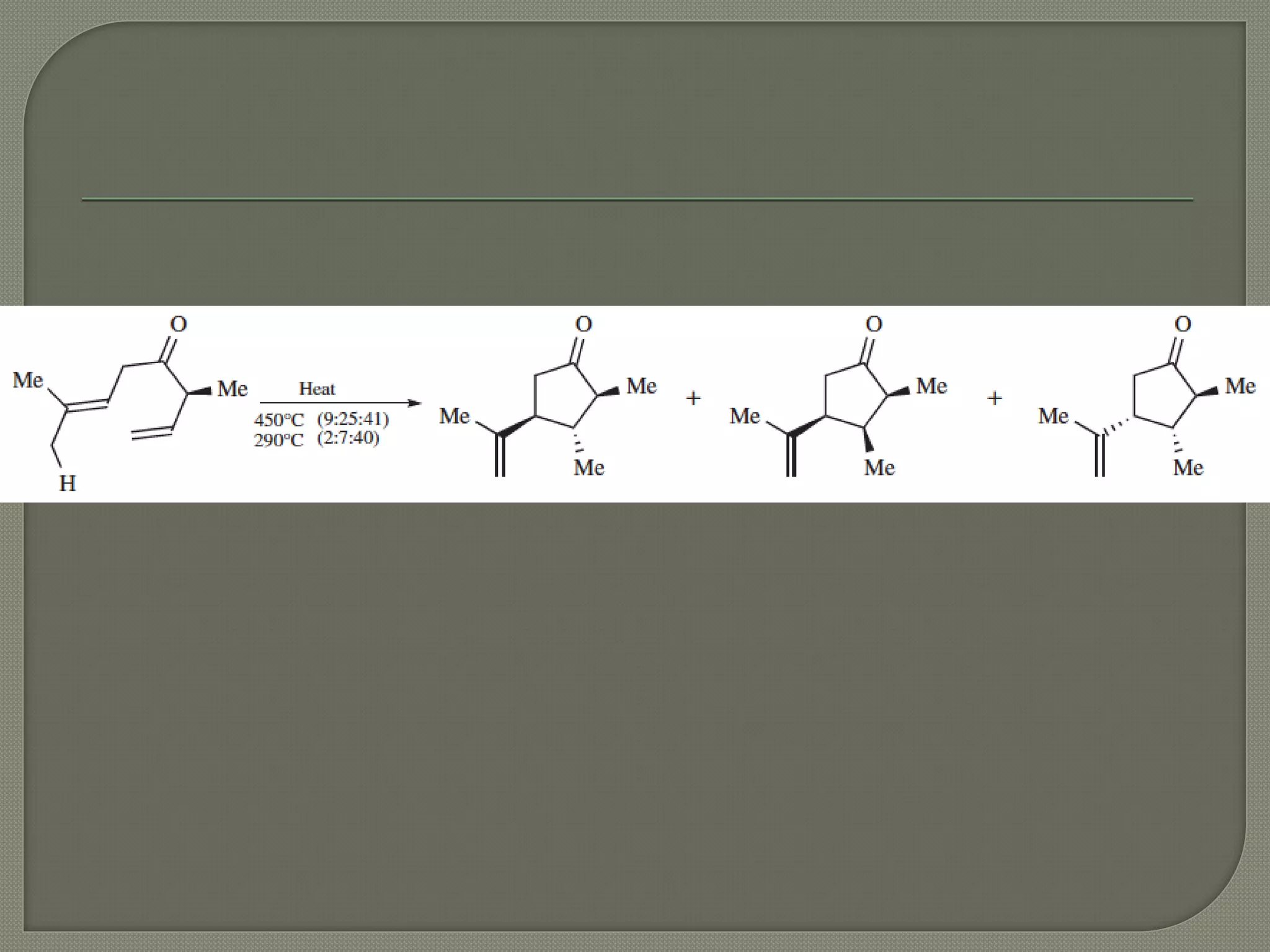
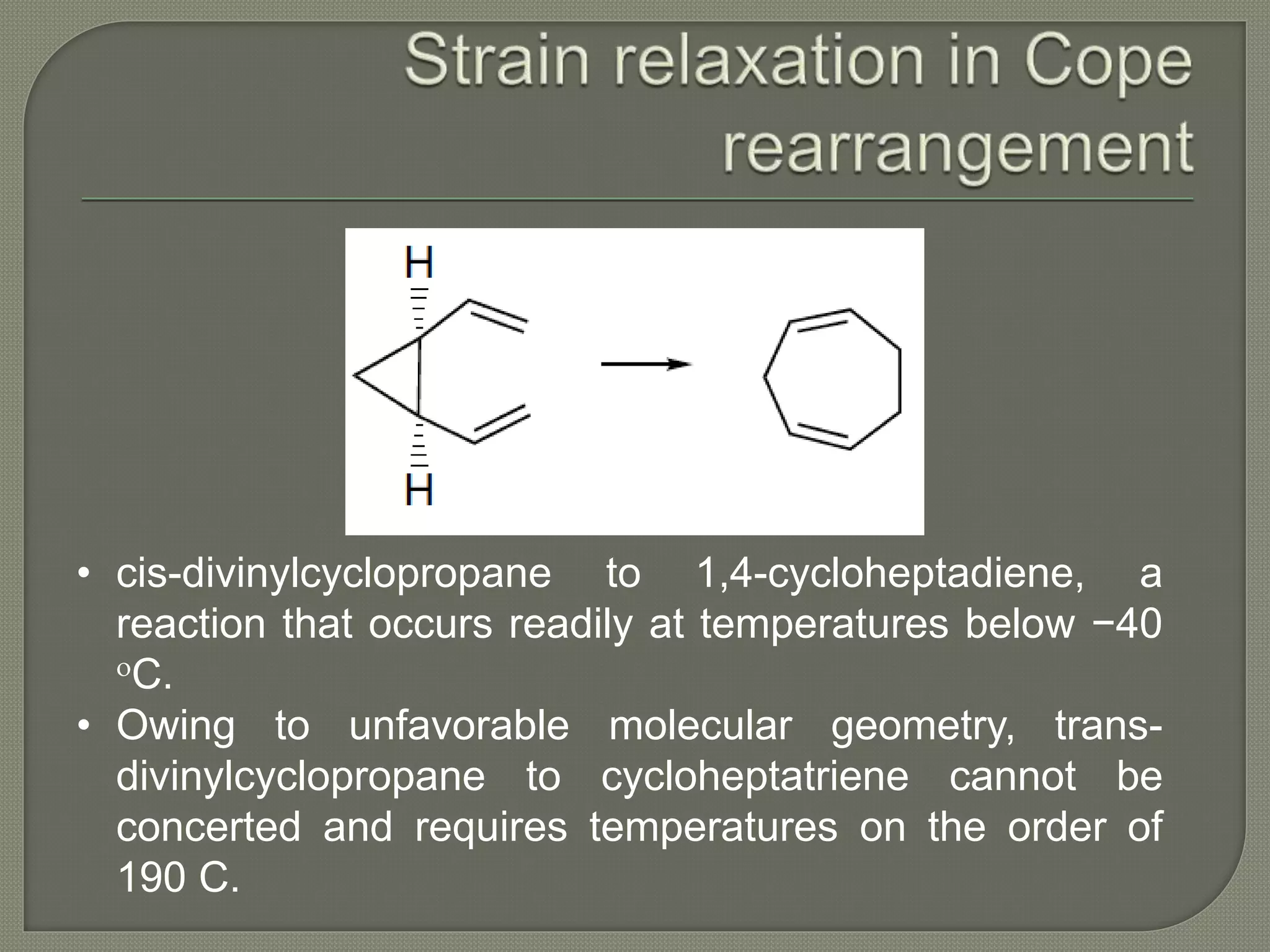
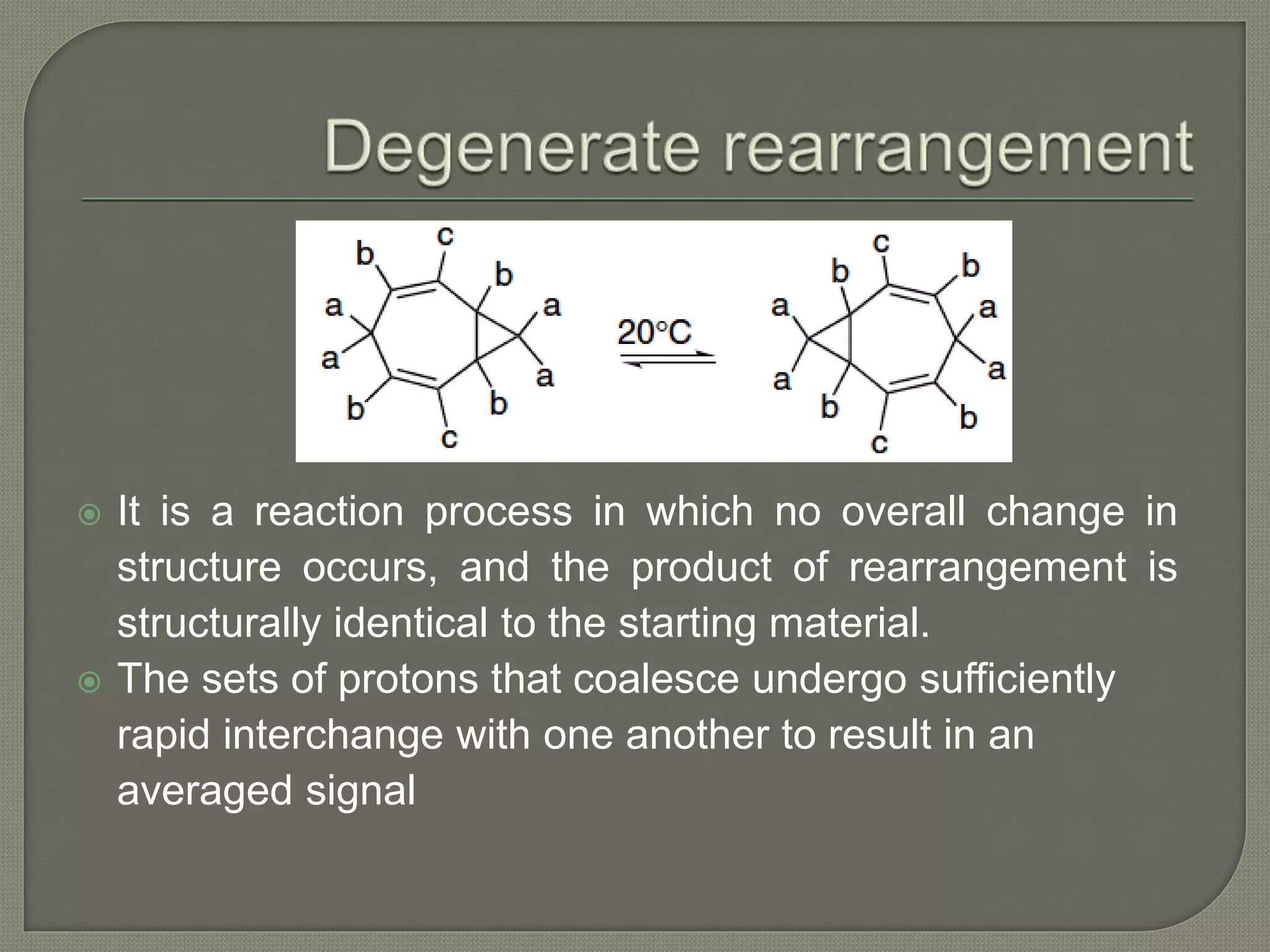
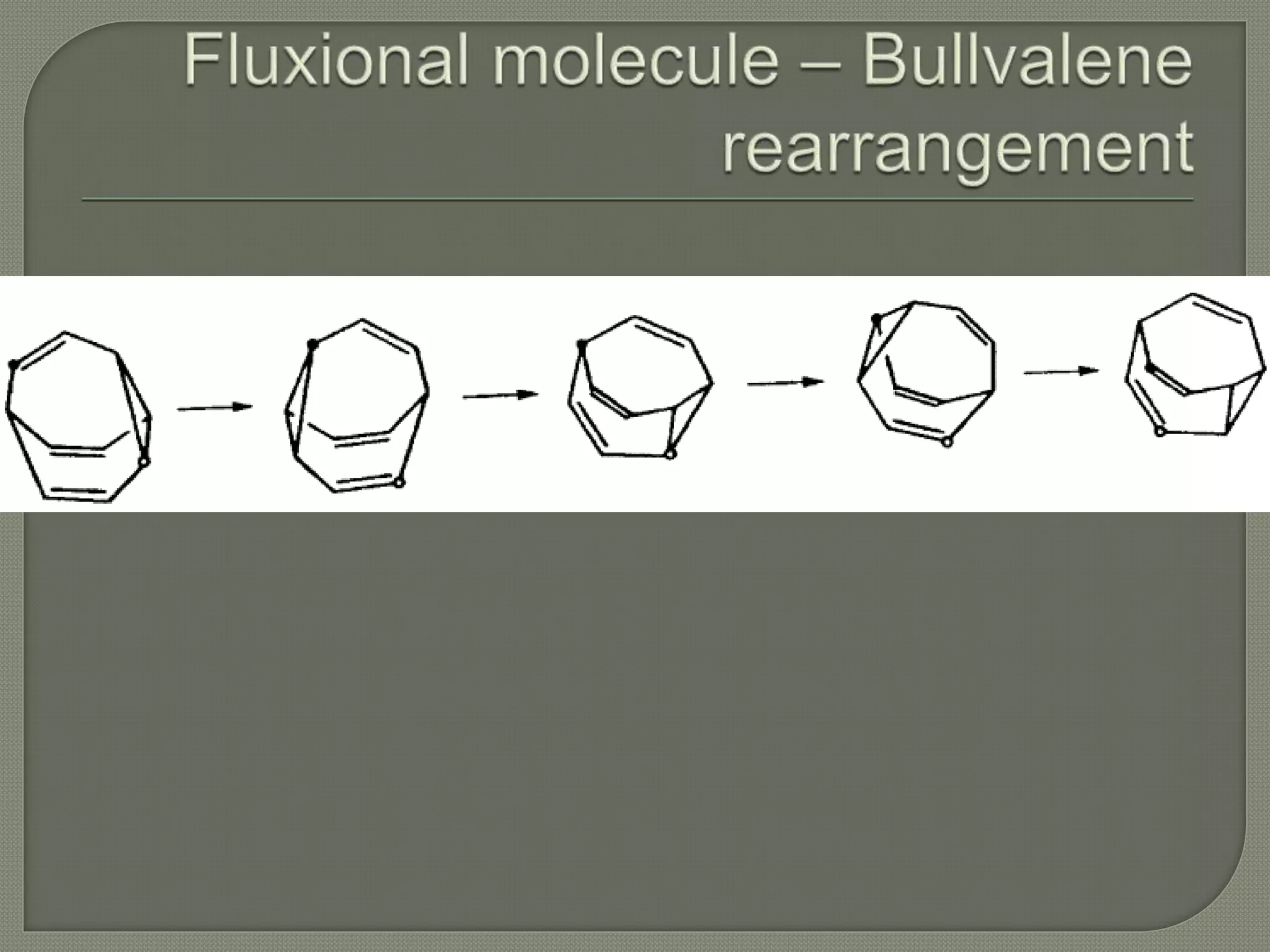
This document discusses pericyclic reactions, including their types, mechanisms, and synthetic importance, particularly in relation to electron reorganization. It covers electrocyclic reactions, cycloaddition reactions, and sigmatropic rearrangements, highlighting examples and the quantum mechanical principles behind these processes. The document also touches on specific reactions like the Diels-Alder and ene reactions, as well as the fluxional nature of bulvalene.