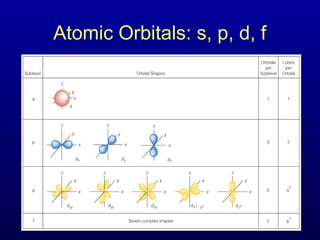
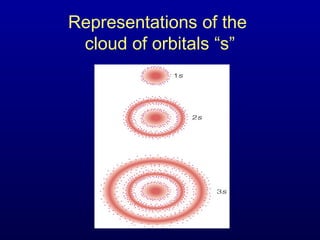
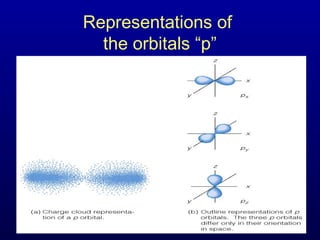
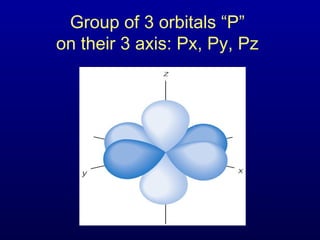
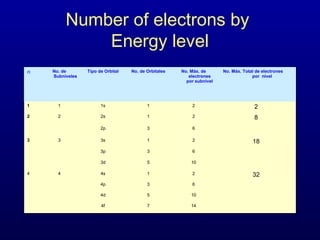
The four quantum numbers describe the distribution and behavior of electrons in atoms. They are derived from the Schrodinger equation for the hydrogen atom. The four quantum numbers are the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (m), and the spin quantum number (s). The principal quantum number determines the electron's energy level. The azimuthal quantum number indicates the subshell and possible shapes of an orbital. The magnetic quantum number describes the orientation of an orbital. The spin quantum number indicates the spin of an electron.