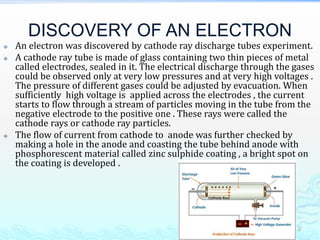
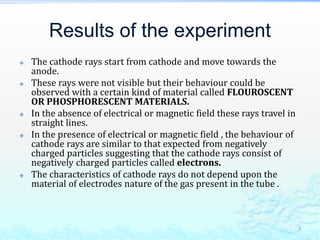
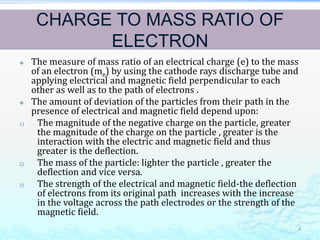
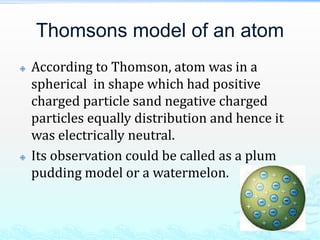
1) Experiments with cathode ray tubes led to the discovery of the electron as a negatively charged fundamental particle.
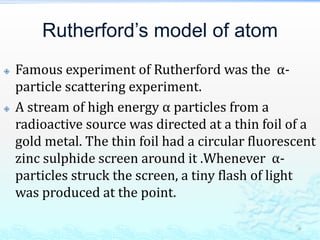
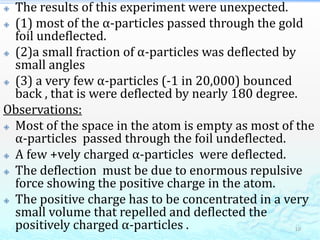
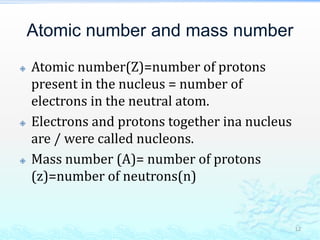
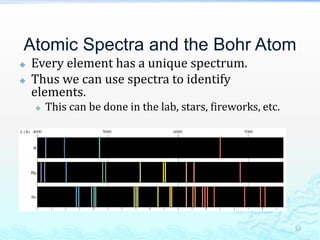
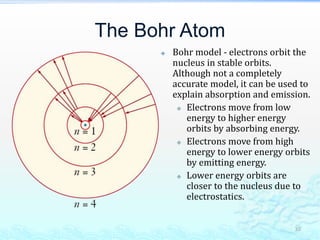
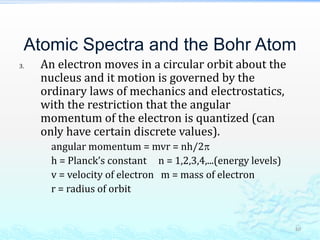
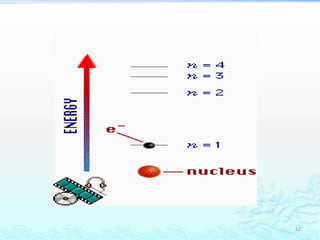
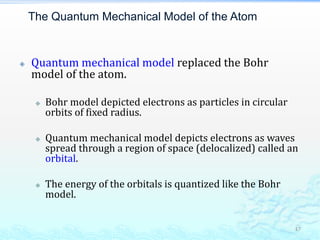
2) Further experiments showed that atoms are mostly empty space and contain a small, dense nucleus made up of protons and neutrons, around which electrons orbit.
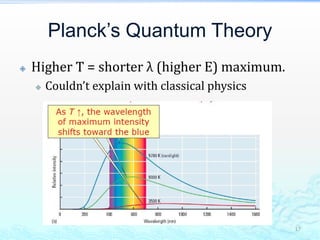
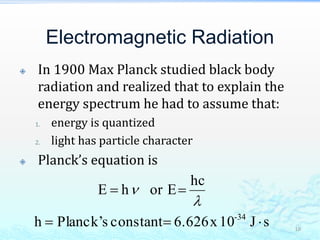
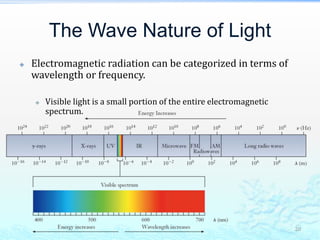
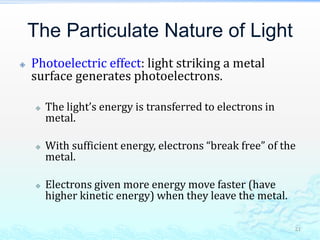
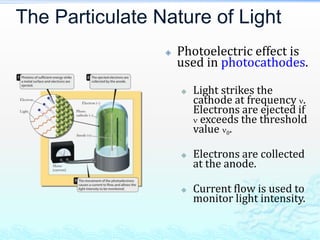
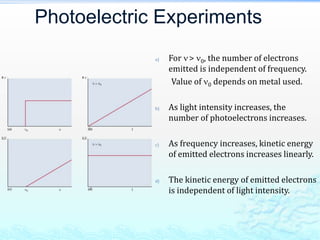
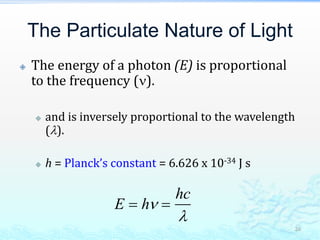
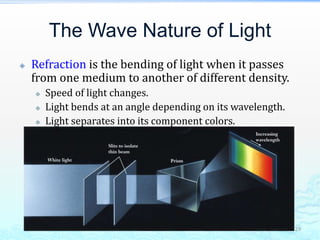
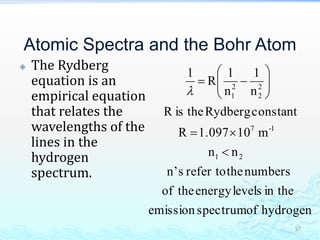
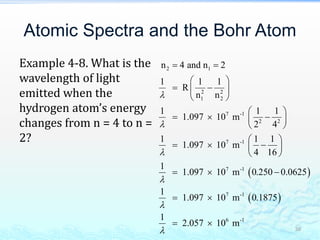
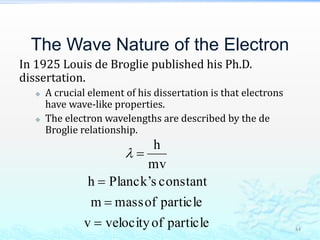
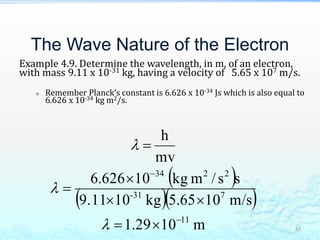
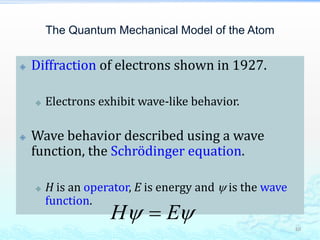
3) The photoelectric effect showed that light behaves as a particle (photon) rather than just a wave, transferring its energy in discrete quantized amounts to electrons and ejecting them from metal surfaces.