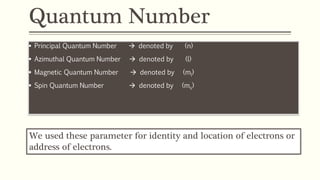
The document discusses the four quantum numbers used to describe electrons in an atom:
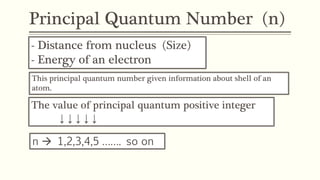
1) The principal quantum number (n) indicates the electron shell and energy level of an electron. Higher n corresponds to higher energy levels further from the nucleus.
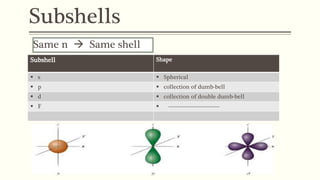
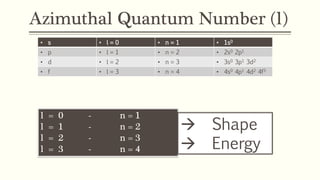
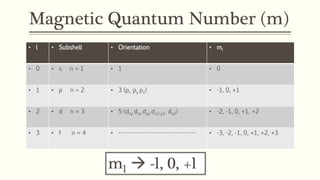
2) The azimuthal quantum number (l) indicates the subshell shape (s, p, d, f) and relates to the orbital angular momentum.
3) The magnetic quantum number (ml) indicates the orientation of orbitals within a subshell and can have values from -l to +l.
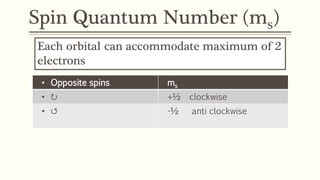
4) The spin quantum number (ms) describes the spin of an electron as +1/2 or -1/2, allowing a maximum of two