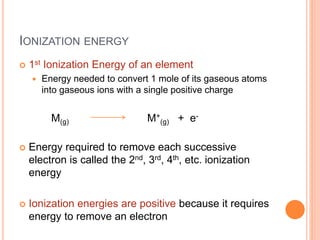
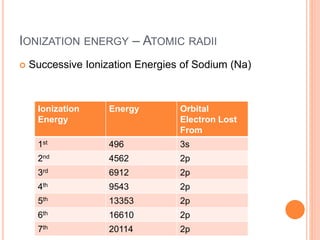
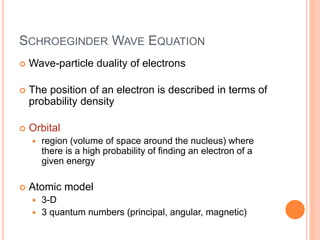
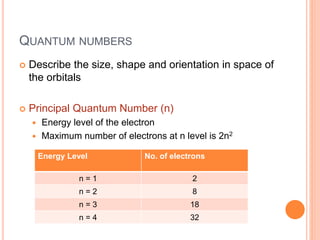
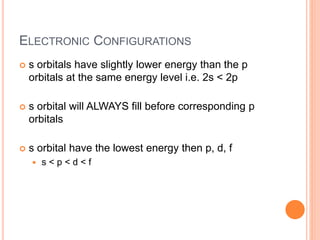
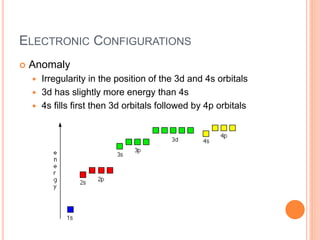
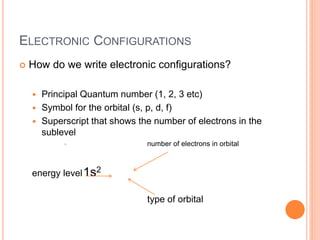
This document discusses quantum numbers and their role in describing the size, shape, and orientation of atomic orbitals. It explains that there are four quantum numbers - principal, angular, magnetic, and spin. The principal quantum number determines the electron shell or energy level, while the angular and magnetic quantum numbers further specify the subshell and orbital within that subshell. The spin quantum number refers to the spin of the electron. Factors that influence ionization energy such as atomic radius, nuclear charge, and electron shielding are also summarized.





![ELECTRONIC CONFIGURATIONS
Atomic Number Symbol Electronic
Configuration
1 H 1s1
2 He 1s2 or [He]
3 Li [He] 2s1
4 Be [He] 2s2
5 B [He] 2s2 2p1
6 C [He] 2s2 2p2
7 N [He] 2s2 2p3
8 O [He] 2s2 2p4
9 F [He] 2s2 2p5
10 Ne [He] 2s2 2p6 or [Ne]](https://image.slidesharecdn.com/quantumnumbers-091011105828-phpapp01/85/Quantum-Numbers-15-320.jpg)
![ELECTRONIC CONFIGURATIONS
Atomic Number Symbol Electronic
Configuration
11 Na [Ne] 3s1
12 Mg [Ne] 3s2
13 Al [Ne] 3s2 3p1
14 Si [Ne] 3s2 3p2
15 P [Ne] 3s2 3p3
16 S [Ne] 3s2 3p4
17 Cl [Ne] 3s2 3p5
18 Ar [Ne] 3s2 3p6 or [Ar]
19 K [Ar] 4s1
20 Ca [Ar] 4s2](https://image.slidesharecdn.com/quantumnumbers-091011105828-phpapp01/85/Quantum-Numbers-16-320.jpg)
![ELECTRONIC CONFIGURATIONS
Atomic Number Symbol Electronic
Configuration
21 Sc [Ar] 4s2 3d1
22 Ti [Ar] 4s2 3d2
23 V [Ar] 4s2 3d3
24 Cr [Ar] 4s1 3d5
25 Mn [Ar] 4s2 3d5
26 Fe [Ar] 4s2 3d6
27 Co [Ar] 4s2 3d7
28 Ni [Ar] 4s2 3d8
29 Cu [Ar] 4s1 3d10
30 Zn [Ar] 4s2 3d10](https://image.slidesharecdn.com/quantumnumbers-091011105828-phpapp01/85/Quantum-Numbers-17-320.jpg)
![ELECTRONIC CONFIGURATIONS –
ABBREVIATED
He, Ne and Ar have electronic configurations with
filled shells of orbitals
Abbreviated electronic configurations
He = 1s2 or [He]
Ne = 1s2 2s2 2p6 or [Ne]
Ar = 1s2 2s2 2p6 3s2 3p6 or [Ar]](https://image.slidesharecdn.com/quantumnumbers-091011105828-phpapp01/85/Quantum-Numbers-18-320.jpg)
![ELECTRONIC CONFIGURATIONS - SPECIAL
After 3p orbitals are filled, 4s orbital is filled before
the 3d orbital
4s orbital is at a slightly lower energy than the 3d
K is [Ar] 4s1
Ca is [Ar] 4s2
Sc is [Ar] 4s2 3d1](https://image.slidesharecdn.com/quantumnumbers-091011105828-phpapp01/85/Quantum-Numbers-19-320.jpg)
![ELECTRONIC CONFIGURATIONS - SPECIAL
After Sc, the 3d orbitals are filled
Irregularity is seen in the electronic configuration of
Cr and Cu
Cr is [Ar] 4s1 3d5
Cu is [Ar] 4s1 3d10](https://image.slidesharecdn.com/quantumnumbers-091011105828-phpapp01/85/Quantum-Numbers-20-320.jpg)