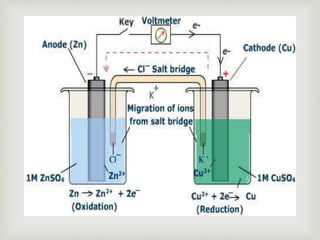
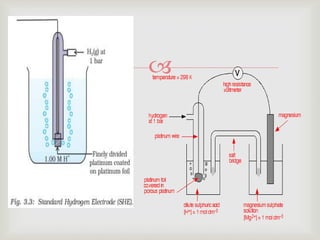
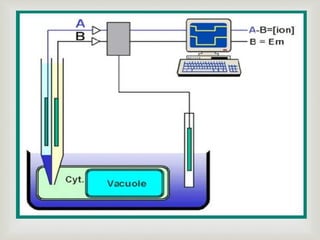
Potentiometry is an electroanalytical technique where the potential difference between two electrodes is measured under conditions of no current flow. It was invented in 1841 by Johann Christian Poggendorff using a slide-wire potentiometer. A potentiometric cell consists of a reference electrode with a known potential and an indicator electrode, whose potential changes depending on the analyte concentration. The potential difference between the electrodes is measured to determine the analyte concentration. Common applications of potentiometry include titrations, analysis of pollutants, drugs, foods, and more.