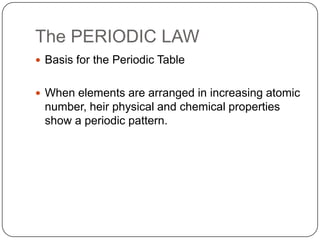
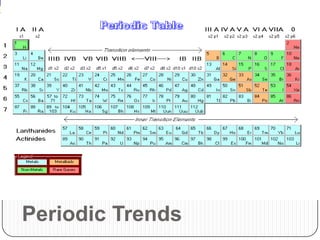
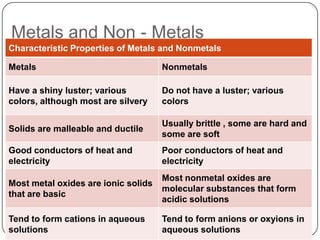
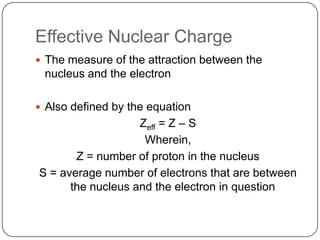
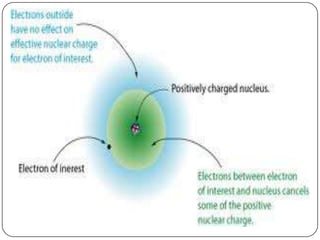
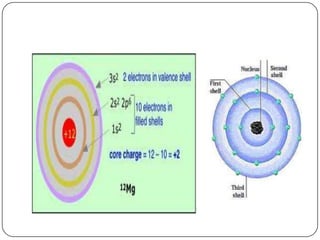
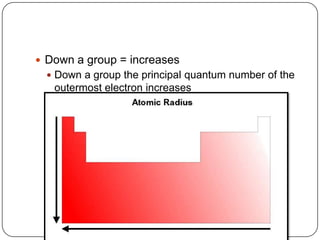
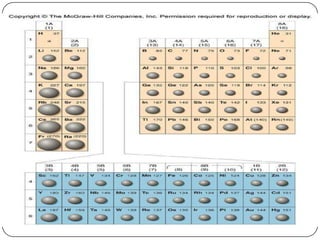
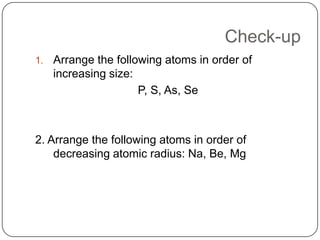
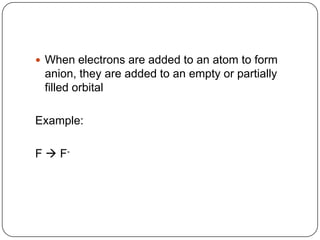
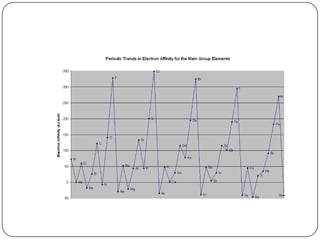
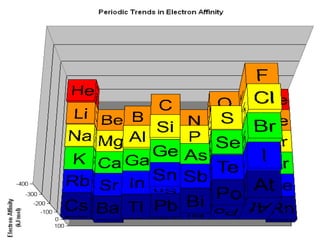
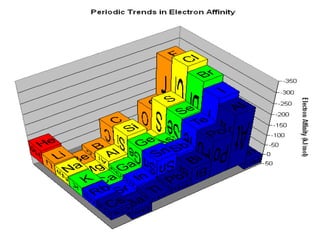
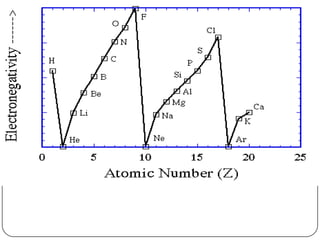
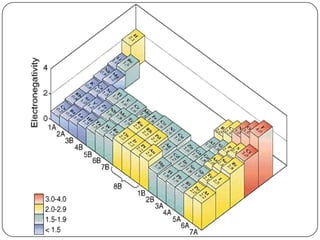
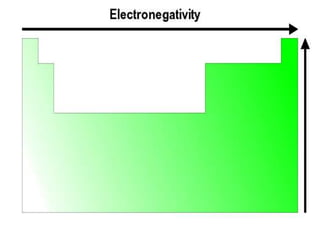
The document summarizes the development of the periodic table of elements from early classifications by scientists like Dobereiner, Newlands, and Mendeleev to the modern periodic table based on atomic number. It describes key periodic properties including atomic size, ionization energy, and electronegativity that led to the establishment of recurring trends in physical and chemical properties of elements when arranged in the periodic table.