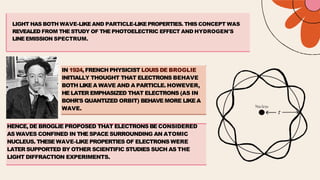
French physicist Louis de Broglie initially proposed that electrons behave as both particles and waves in 1924. Erwin Schrödinger further developed this idea with a wave equation to describe electron behavior in 1926. Quantum theory explains the mathematical description of electron wave properties and orbital probabilities, superseding the classical view of electrons orbiting the nucleus along precise paths. It became the basis of modern atomic structure models.






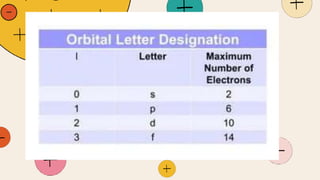

![Magnetic Quantum Number
The magnetic quantum number (m l ) indicates the orientation of an orbital around the nucleus.
for a particular value of l, there will be (2l+1) possible values of m l. Hence, the values for m l are
integers from -l to +l , including 0. for example, i l = 0, only one value for ml is possible : that is ml=0.
If l=1, there are [2l +1 = 3] possible values of ml, which are -1, 0 and +1.
The number of ml values also gives an idea about the number of orientations of orbital belonging
to a particular subshell. for instance, for a p orbital with l=m1, the three possible ml values (-1,0,+1
) imply that there are three orientations of the p orbital around the nucleus.](https://image.slidesharecdn.com/quantummodel-240227022858-0bba5fcb/85/QUANTUM-MODEL-grade11-pptx-10-320.jpg)