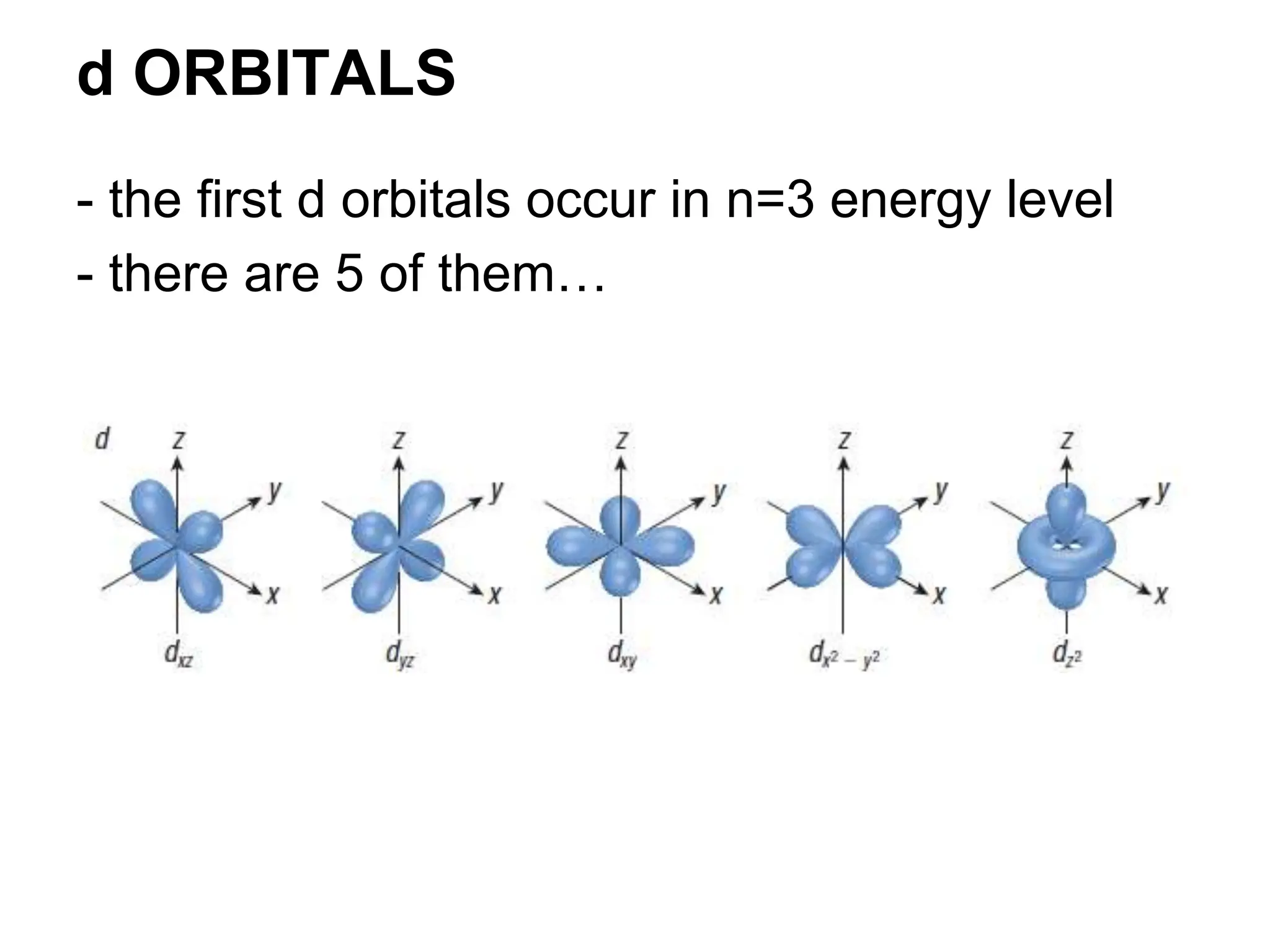
Quantum numbers are essential for describing the position and energy of electrons in an atom, consisting of four types: principal, subsidiary, magnetic, and spin quantum numbers. They define energy levels, shapes, orientations of orbitals, and the behavior of electrons as described by principles such as the Pauli exclusion principle, Hund's rule, and the Aufbau principle. Each quantum number plays a critical role in understanding electron configurations and the structure of atoms.