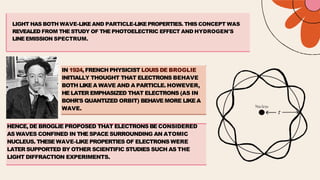
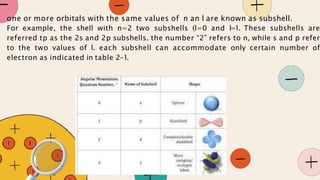
French physicist Louis de Broglie initially proposed that electrons behave as both particles and waves in 1924. Erwin Schrödinger further developed this idea with a wave equation to describe electron behavior in 1926. Quantum theory explains the mathematical description of electron wave properties and orbital probabilities, revolutionizing the understanding of atomic structure.





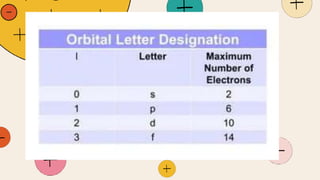
![Magnetic Quantum Number
The magnetic quantum number (m l ) indicates the orientation of an orbital around the nucleus.
for a particular value of l, there will be (2l+1) possible values of m l. Hence, the values for m l are
integers from -l to +l , including 0. for example, i l = 0, only one value for ml is possible : that is ml=0.
If l=1, there are [2l +1 = 3] possible values of ml, which are -1, 0 and +1.
The number of ml values also gives an idea about the number of orientations of orbital belonging
to a particular subshell. for instance, for a p orbital with l=1, the three possible ml values (-1,0,+1 )
imply that there are three orientations of the p orbital around the nucleus.](https://image.slidesharecdn.com/quantummodel-240122234053-084bd60d/85/QUANTUM-MODEL-pptx-10-320.jpg)