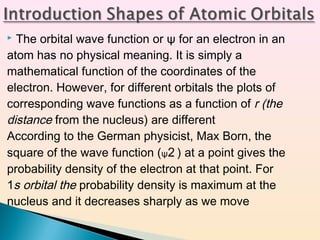
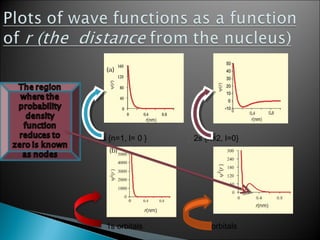
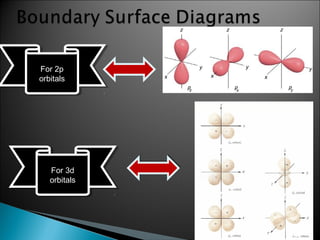
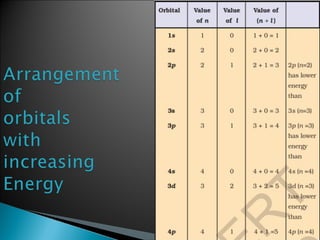
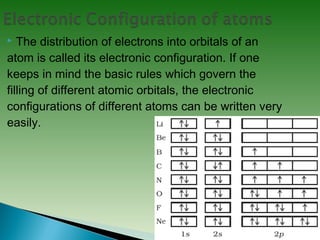
Classical mechanics successfully describes macroscopic objects but fails for microscopic objects like atoms and molecules. Quantum mechanics was developed to account for these microscopic objects, which exhibit both wave-like and particle-like properties. It describes that electrons can have distinct quantum states defined by quantum numbers like principal (n), azimuthal (l), magnetic (m), and spin (s). The values of these quantum numbers determine properties of atomic orbitals like shape, size, and energy.





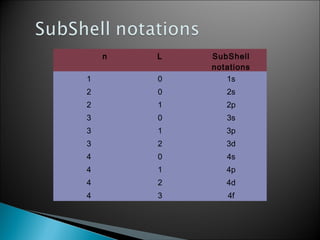
![ Magnetic orbital quantum number. ‘m’ gives information about the spatial orientation ofMagnetic orbital quantum number. ‘m’ gives information about the spatial orientation of
thethe
orbital with respect to standard set of co-ordinate axis. For any sub-shell (defined by ‘l’orbital with respect to standard set of co-ordinate axis. For any sub-shell (defined by ‘l’
value)value)
2l+1 values of m are possible and these values are given by : m2l+1 values of m are possible and these values are given by : m11 = – l, – (l –1), – (l –2)... 0,1...= – l, – (l –1), – (l –2)... 0,1...
(l – 2), (l–1), l.(l – 2), (l–1), l.
for l = 0, the only permitted value of mfor l = 0, the only permitted value of mll = 0, [2(0)+1 = 1, one s orbital]. For can be –1, 0 and= 0, [2(0)+1 = 1, one s orbital]. For can be –1, 0 and
+1 [2(1)+1 = 3, three orbitals]. For l = 2, m = –2, –1, 0, +1 and +2, [2(2)+1 = 5, five d orbitals]. It+1 [2(1)+1 = 3, three orbitals]. For l = 2, m = –2, –1, 0, +1 and +2, [2(2)+1 = 5, five d orbitals]. It
should be noted that the values of mshould be noted that the values of mll are derived from that the value of l are derived fromare derived from that the value of l are derived from
n.n.
Value of l 0 1 2 3 4 5
Subshell notation s p d f g h
number of orbitals 1 3 5 7 9 11](https://image.slidesharecdn.com/quantummechanicalmodelofatom-130822000531-phpapp02/85/CBSE-Class-XI-Chemistry-Quantum-mechanical-model-of-atom-7-320.jpg)