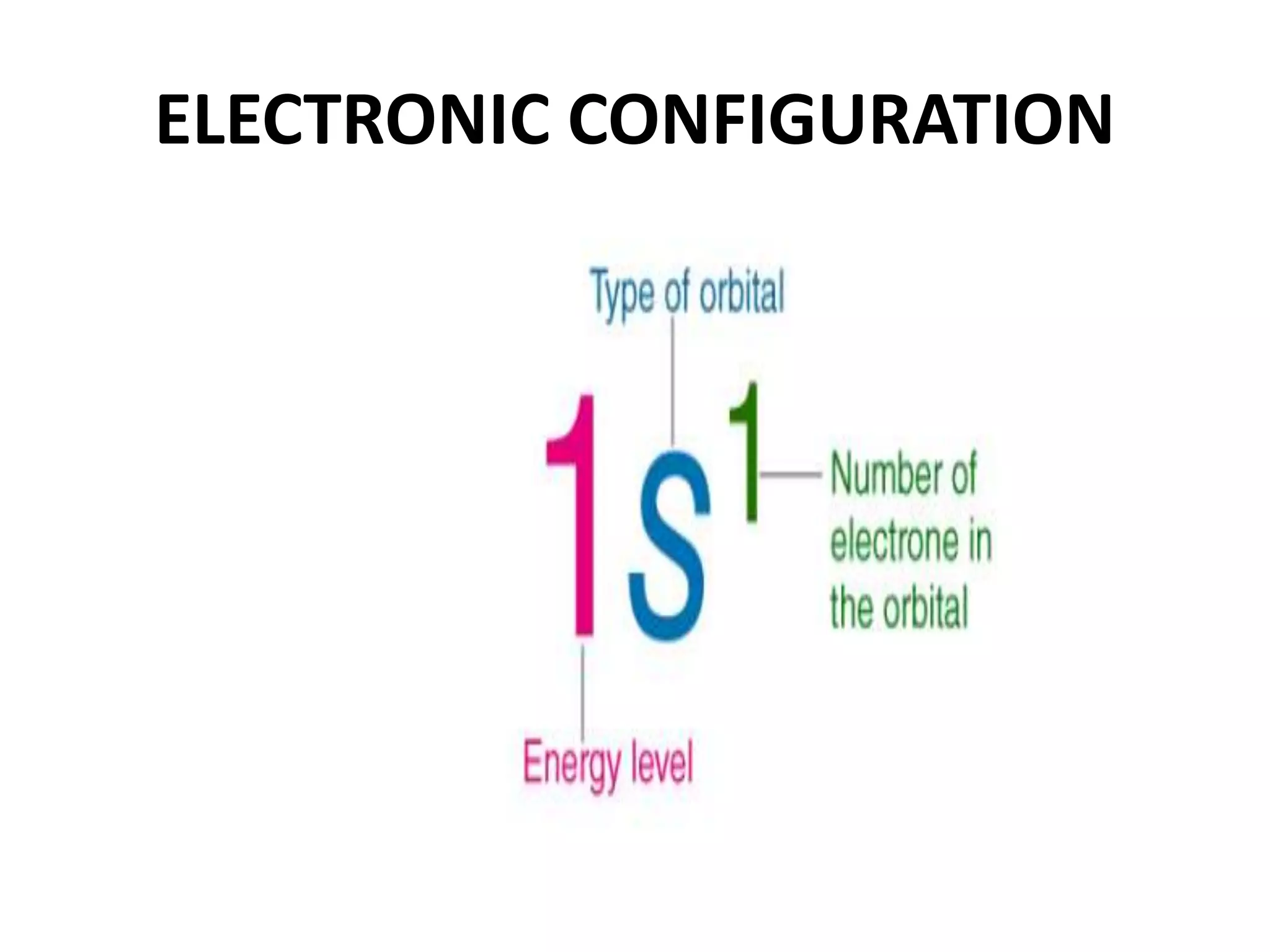
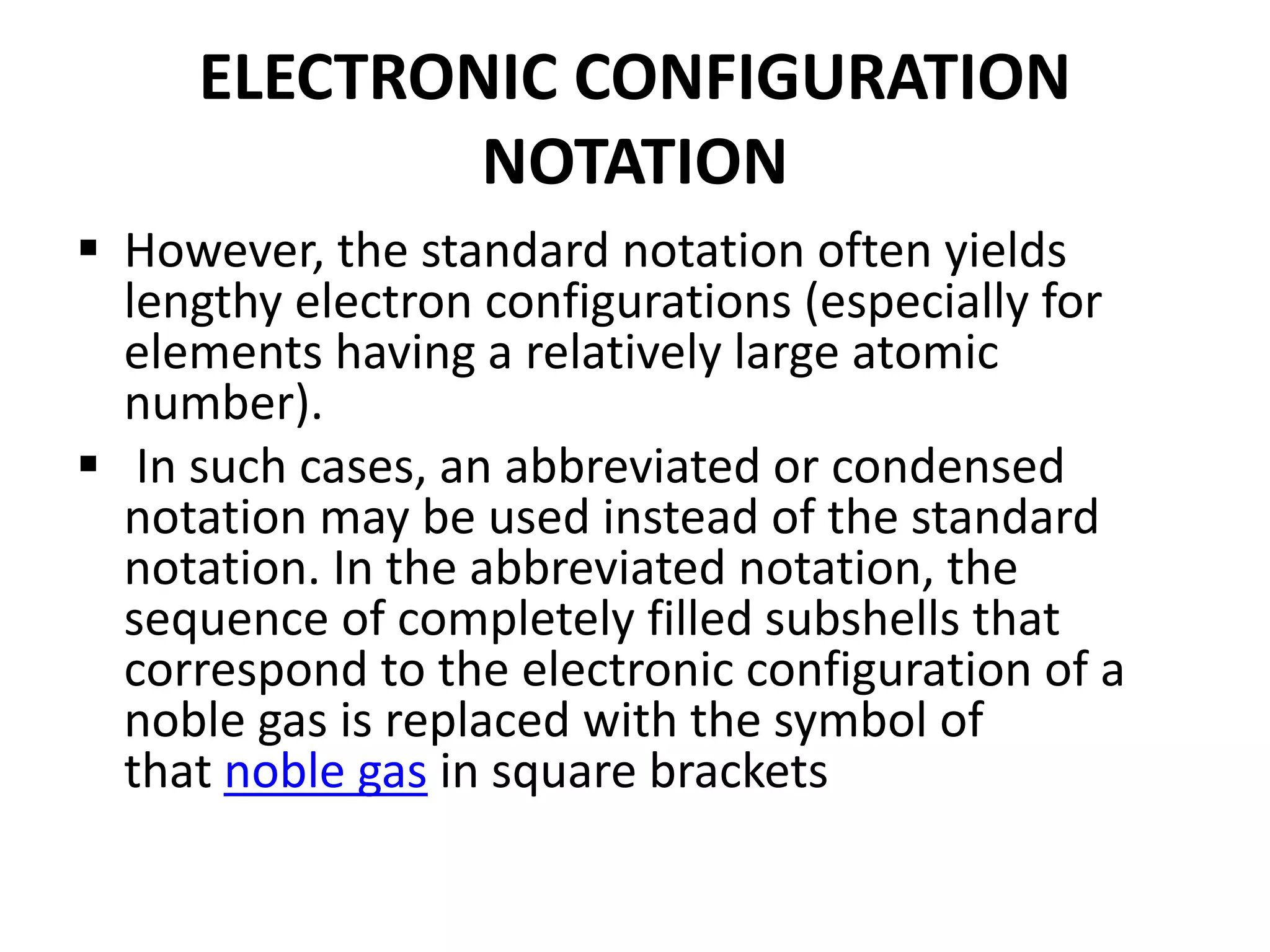
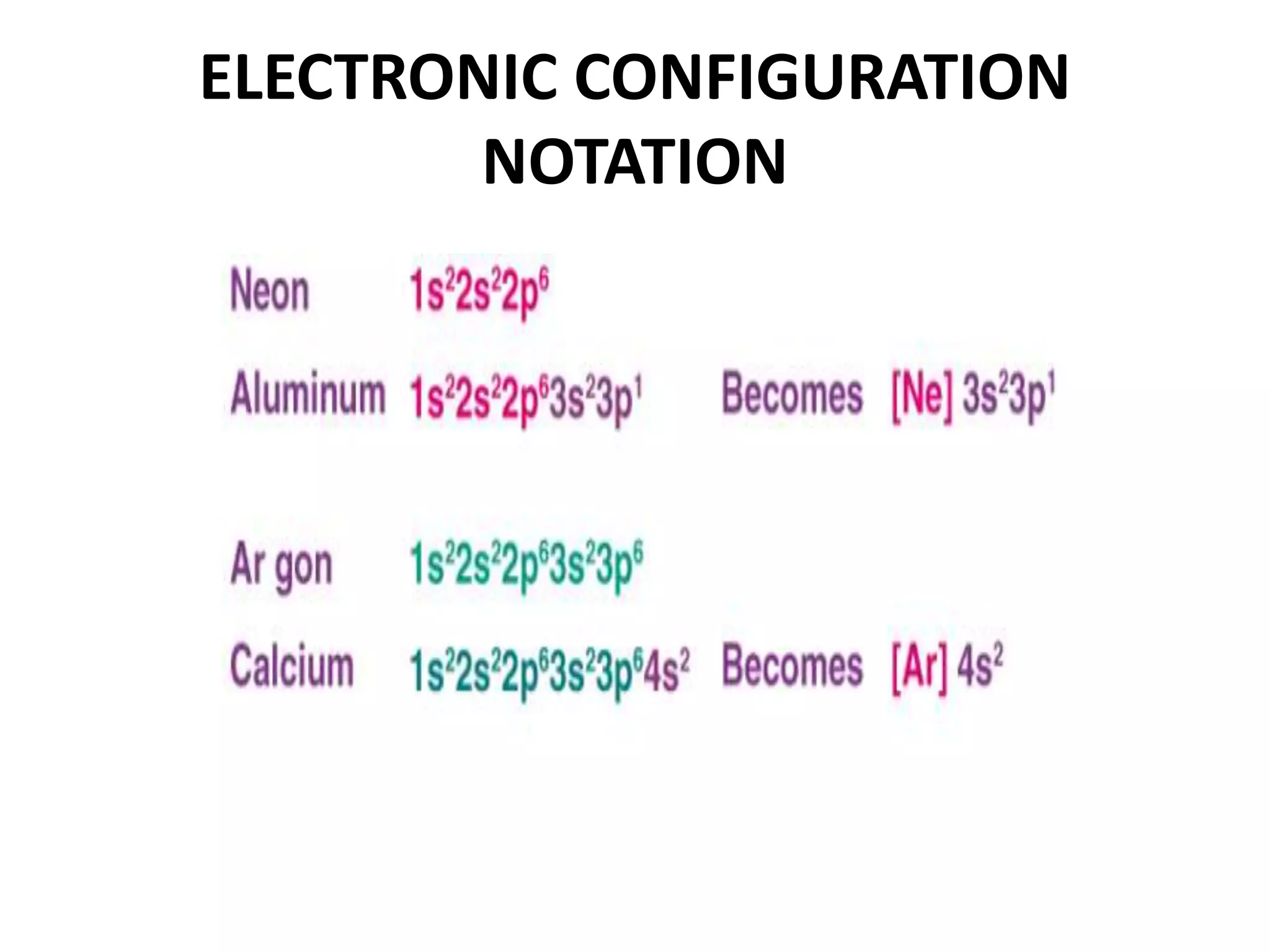
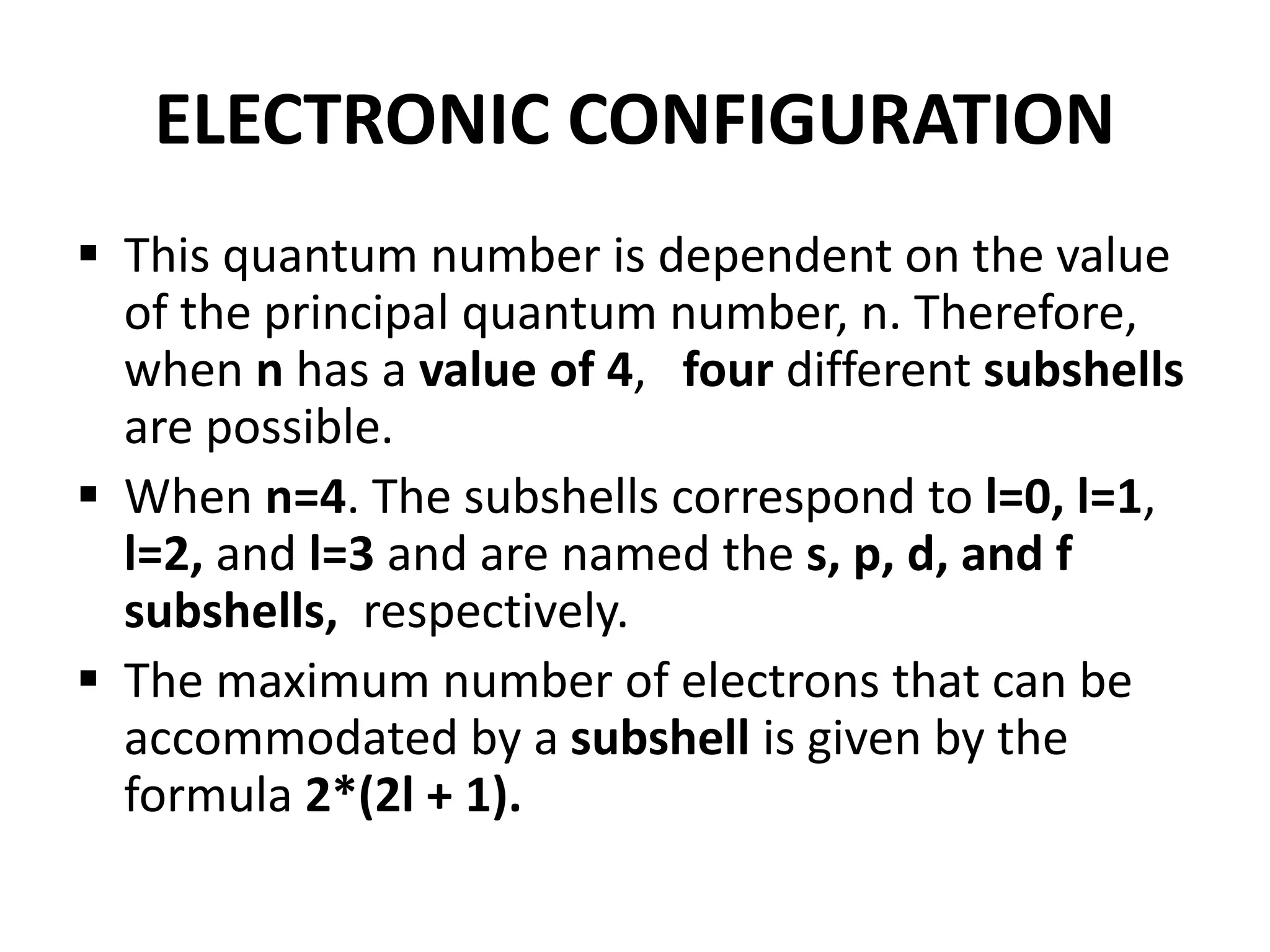
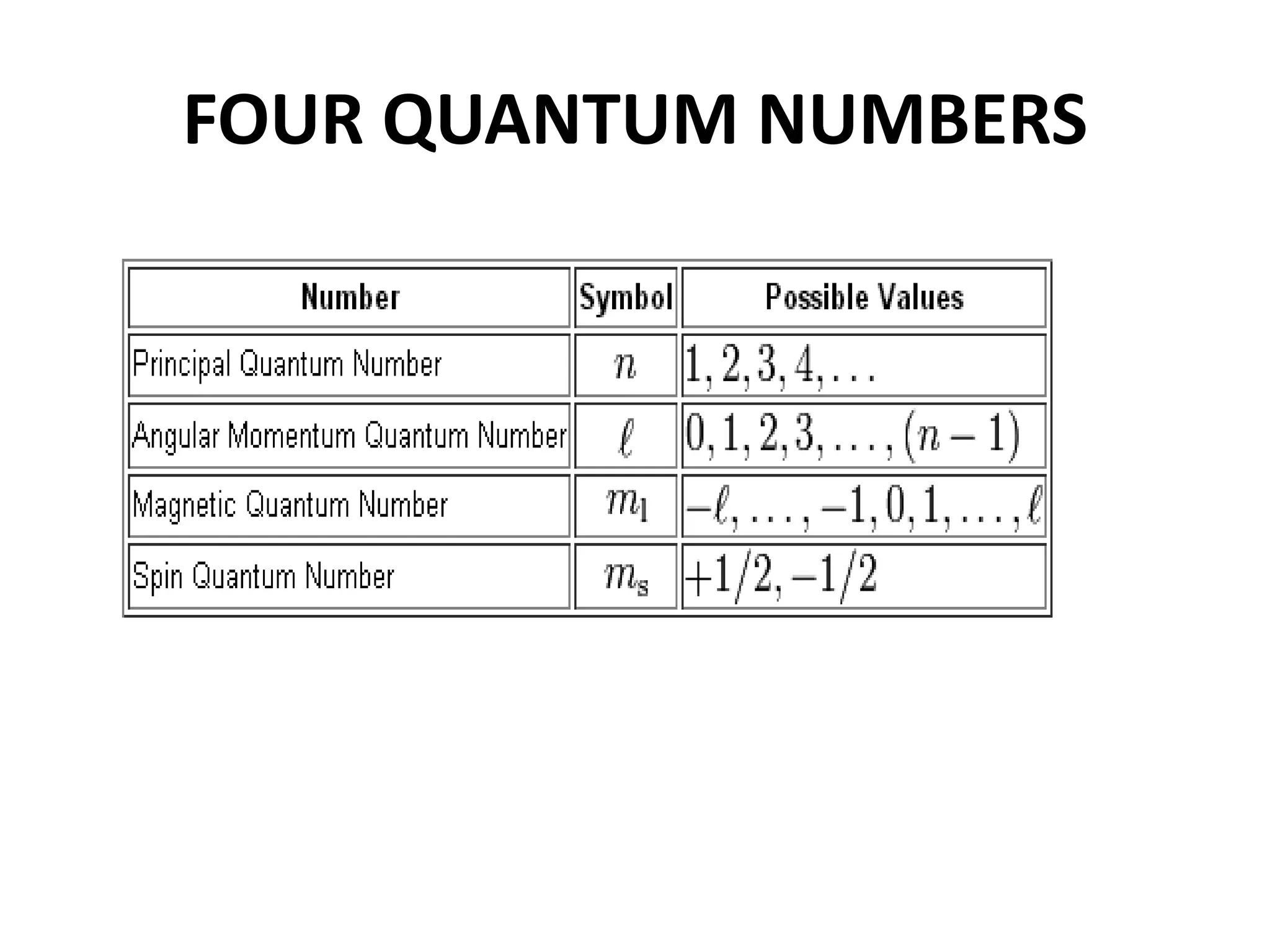
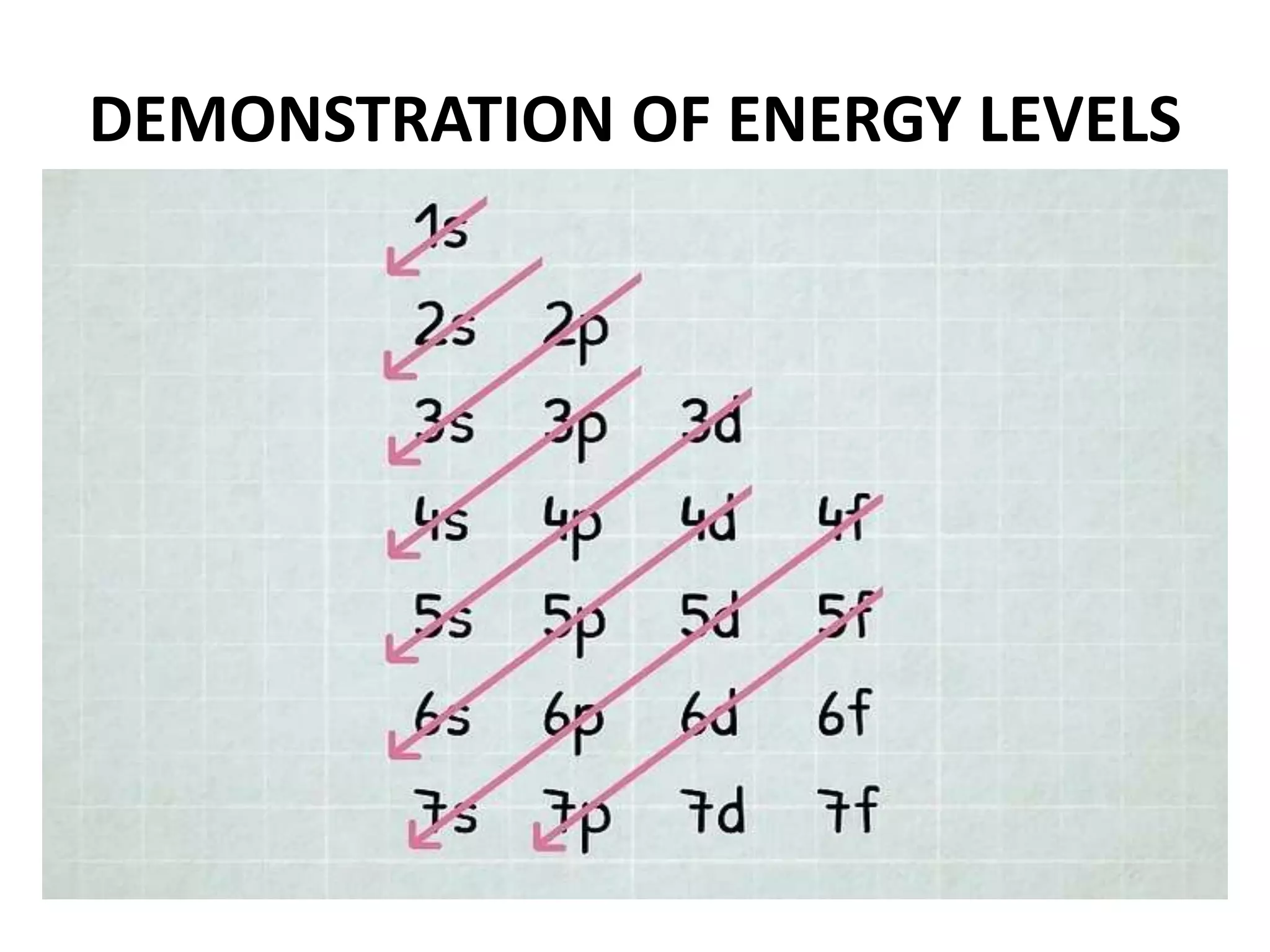
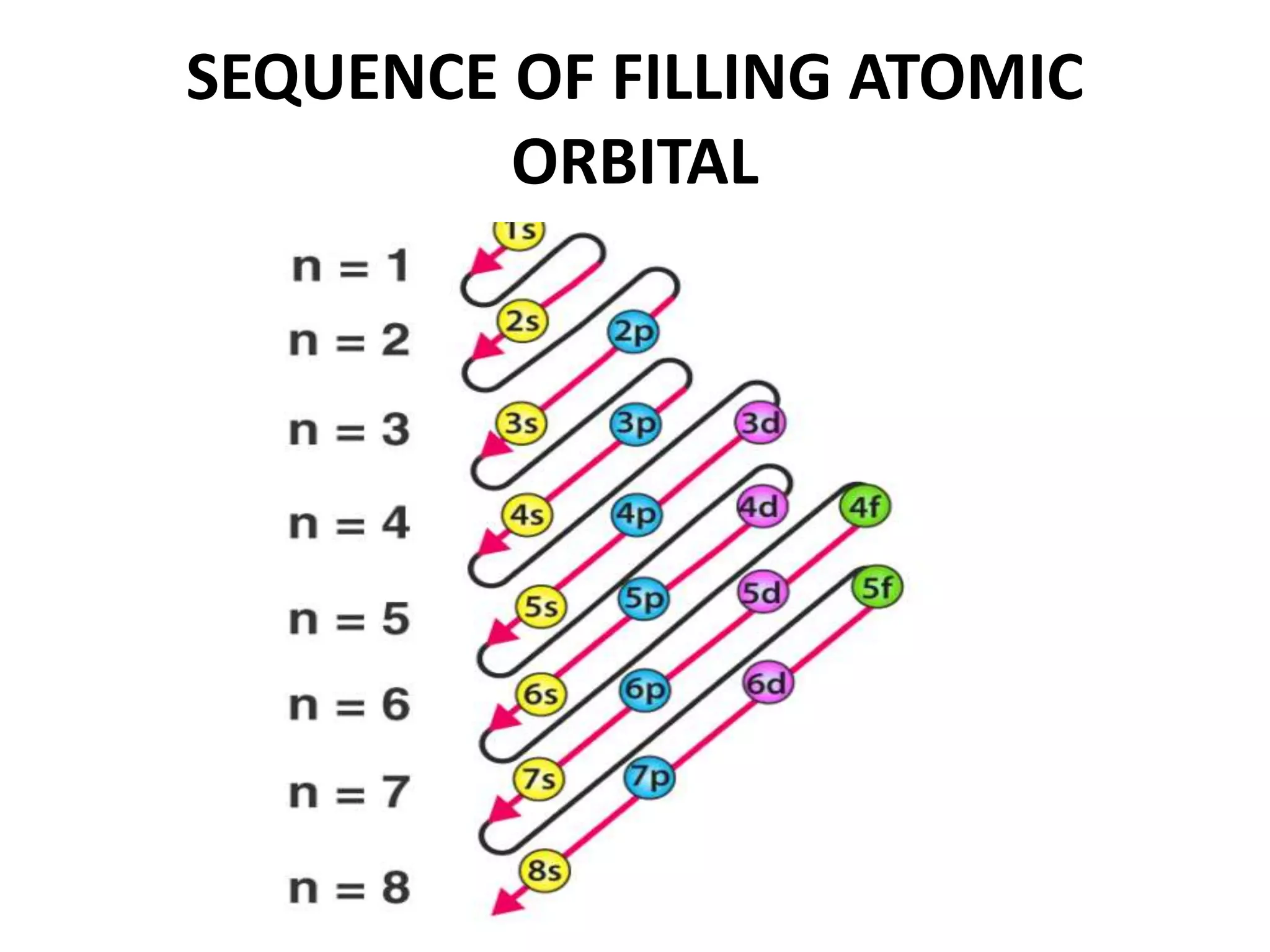
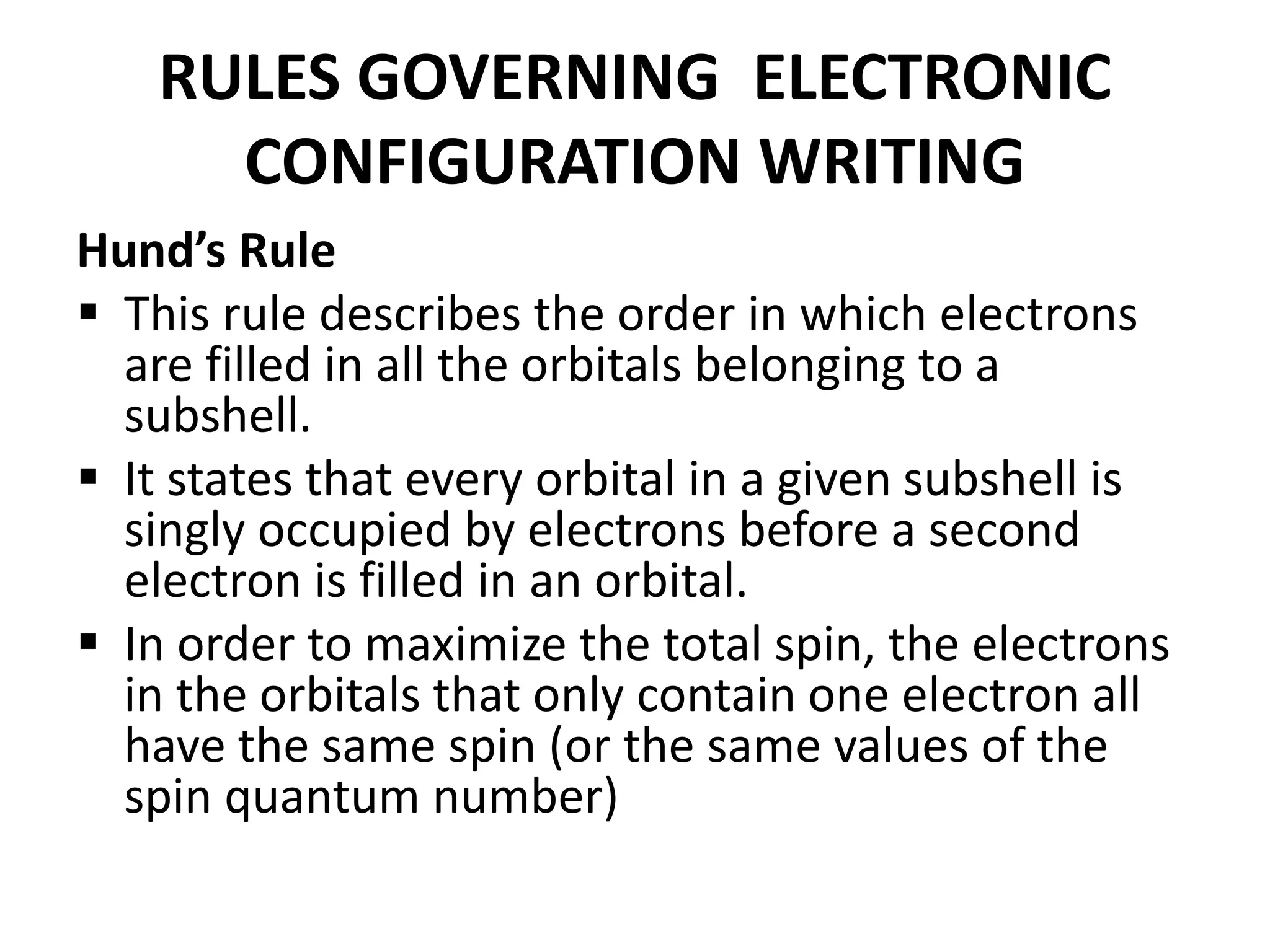
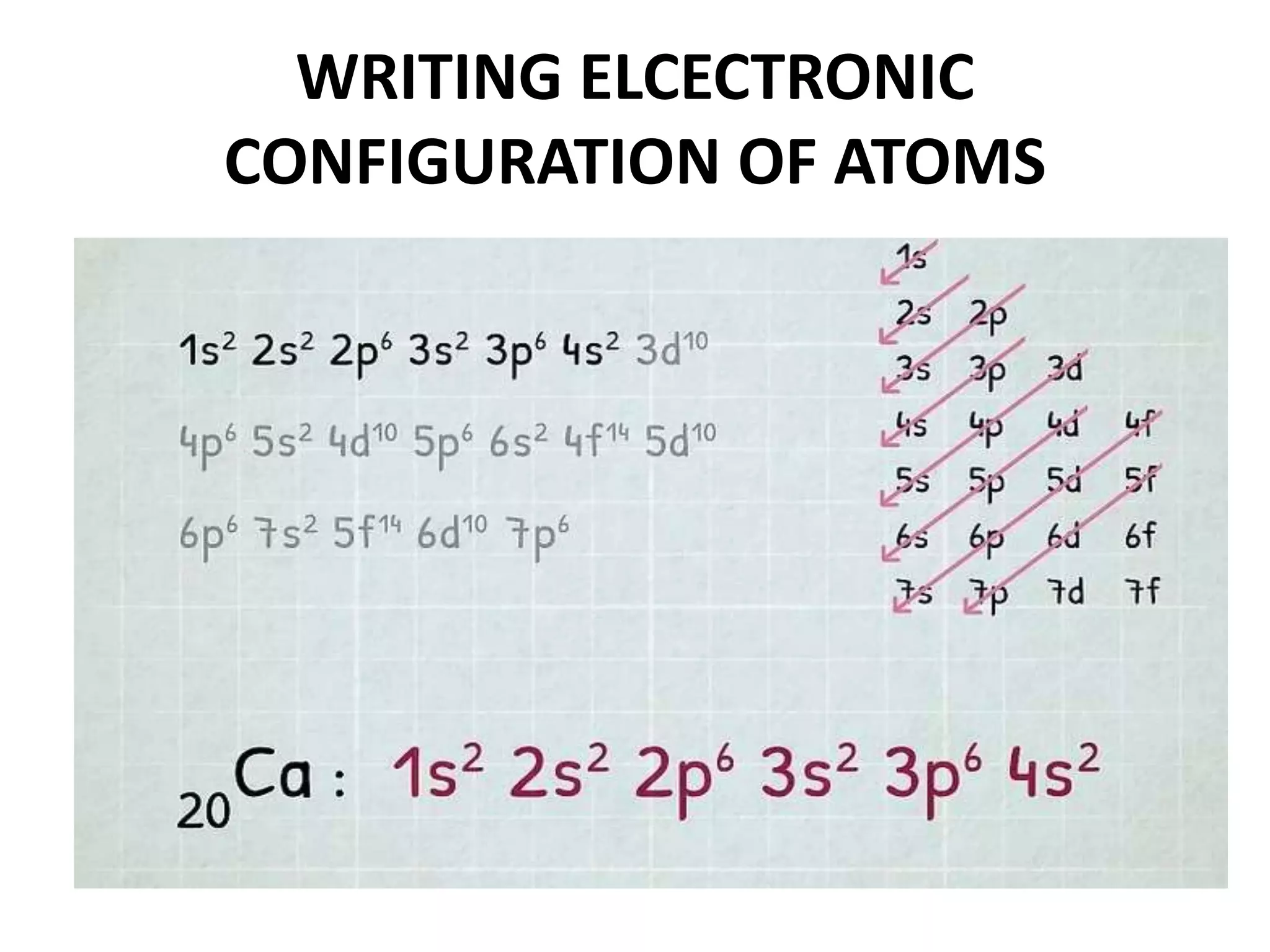
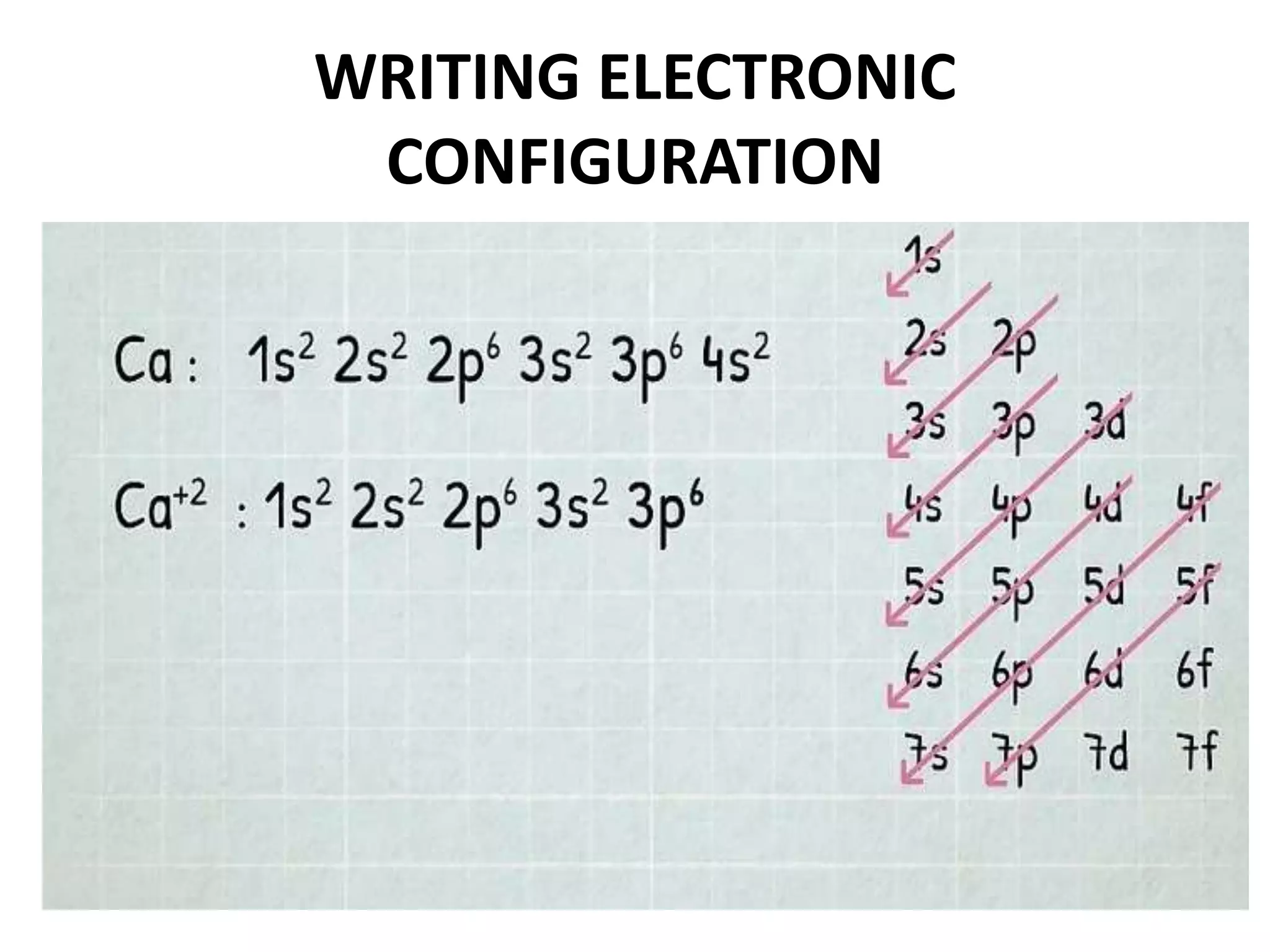
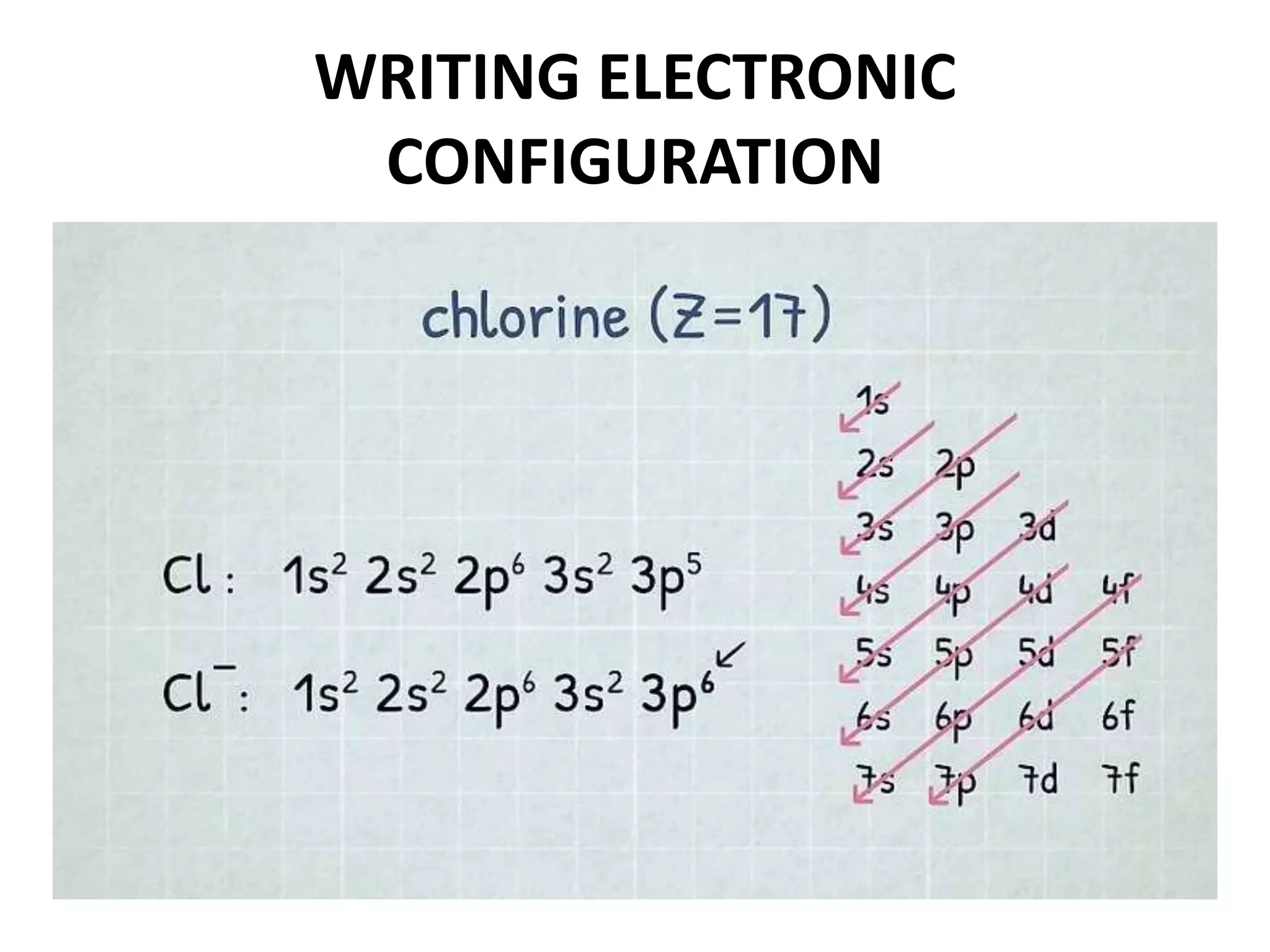
This document provides an overview of electronic configuration and the rules for writing the electronic configurations of elements. It defines electronic configuration as describing how electrons are distributed in an atom's atomic orbitals. It discusses the quantum numbers that describe atomic orbitals and electrons, including the principal, azimuthal, magnetic, and spin quantum numbers. It also explains the Aufbau principle, Pauli exclusion principle, and Hund's rule that govern the filling of electrons in orbitals to write electronic configurations. The goal is for students to understand these concepts and be able to write the electronic configuration of different elements.