This document provides information on electrochemistry and electrochemical cells. It defines electrochemistry as the study of electricity production from spontaneous chemical reactions and use of electrical energy for non-spontaneous reactions. It describes different types of electrochemical cells including galvanic cells that convert chemical to electrical energy and electrolytic cells that do the opposite. Key concepts discussed include electrode potentials, standard hydrogen electrode, Nernst equation, and factors affecting cell potential. Common electrochemical devices like batteries and the corrosion process are also summarized.







![E cell is written as :
E cell = E right – Eleft
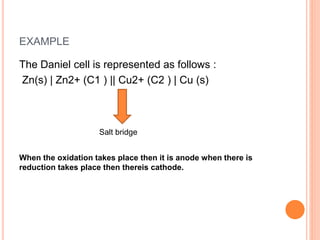
EXAMPLE:-
Cell reaction:
Cu(s) + 2Ag+ (aq) → Cu2+(aq) + 2 Ag(s)
Half-cell reactions:
Cathode (reduction): 2Ag+ (aq) + 2e– → 2Ag(s)
Anode (oxidation): Cu(s) → Cu2+(aq) + 2e
The cell can be represented as:
Cu(s)|Cu2+(aq)||Ag+ (aq)|Ag(s)
Ecell = Eright – Eleft
=E Ag+/ Ag – E Cu2+ /Cu
NOTES:-It is not possible to determine the absolute value
of electrode potential. For this a reference electrode [NHE
or SHE] is required.](https://image.slidesharecdn.com/class12eleetcro-240105142217-7f973e44/85/CLASS-12-ELECTROCHEMISTRY-pptx-12-320.jpg)


































![(e)Calculate the degree of dissociation (α) of acetic acid if its molar
conductivity (∧m) is 39.05 S cm2mol–1. Given λo (H+) = 349.6 S cm2
mol–1 and λo(CH3COO–) = 40.9 S cm2mol–1.
(f) Calculate the mass of Ag deposited at cathode when a current of 2
amperes was passed through a solution of AgNO3 for 15 minutes. (Given
: Molar mass of Ag = 108 g mol–1 1F = 96500 C mol–1)
(g) Define fuel cell.
(h) Write the cell reaction and calculate the e.m.f. of the following cell at 298
K :
Sn (s) | Sn2+ (0·004 M) || H+(0·020 M) | H2(g) (1 bar) | Pt (s) (Given :E0
Sn2+/Sn = – 0·14 V)
(i) Give reasons :
On the basis of Eo values, O2 gas should be liberated at anode but it is
Cl2 gas which is liberated in the electrolysis of aqueous NaCl.
Conductivity of CH3COOH decreases on dilution.
(j) For the reaction 2AgCl (s) + H2 (g) (1 atm) 2Ag (s) + 2H+ (0·1 M)
+ 2Cl–(0·1 M),
∆Go= – 43600 J at 25 C. Calculate the e.m.f. of the cell. [log 10–n= – n]
(k) Define fuel cell and write its two advantages.](https://image.slidesharecdn.com/class12eleetcro-240105142217-7f973e44/85/CLASS-12-ELECTROCHEMISTRY-pptx-47-320.jpg)

