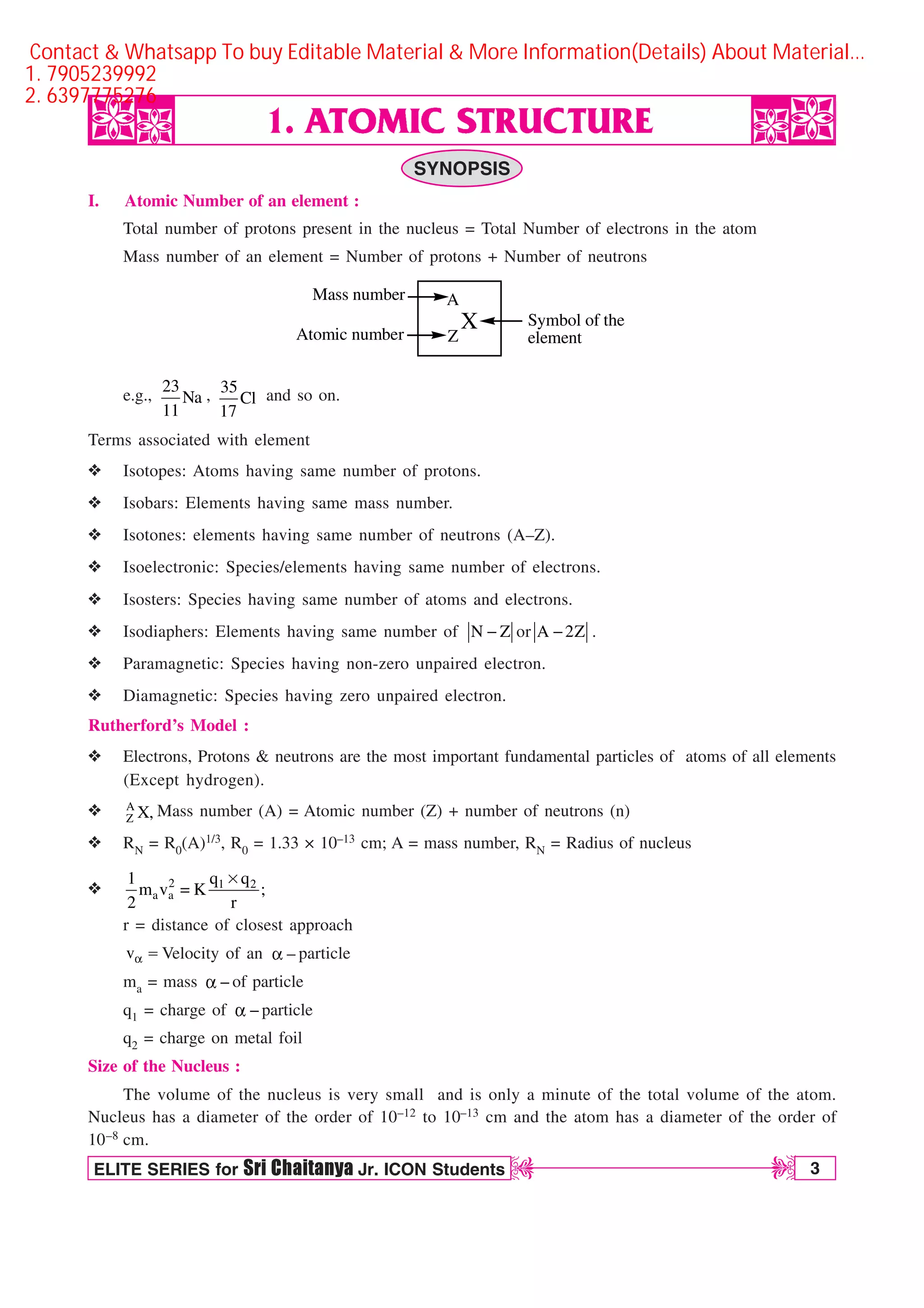
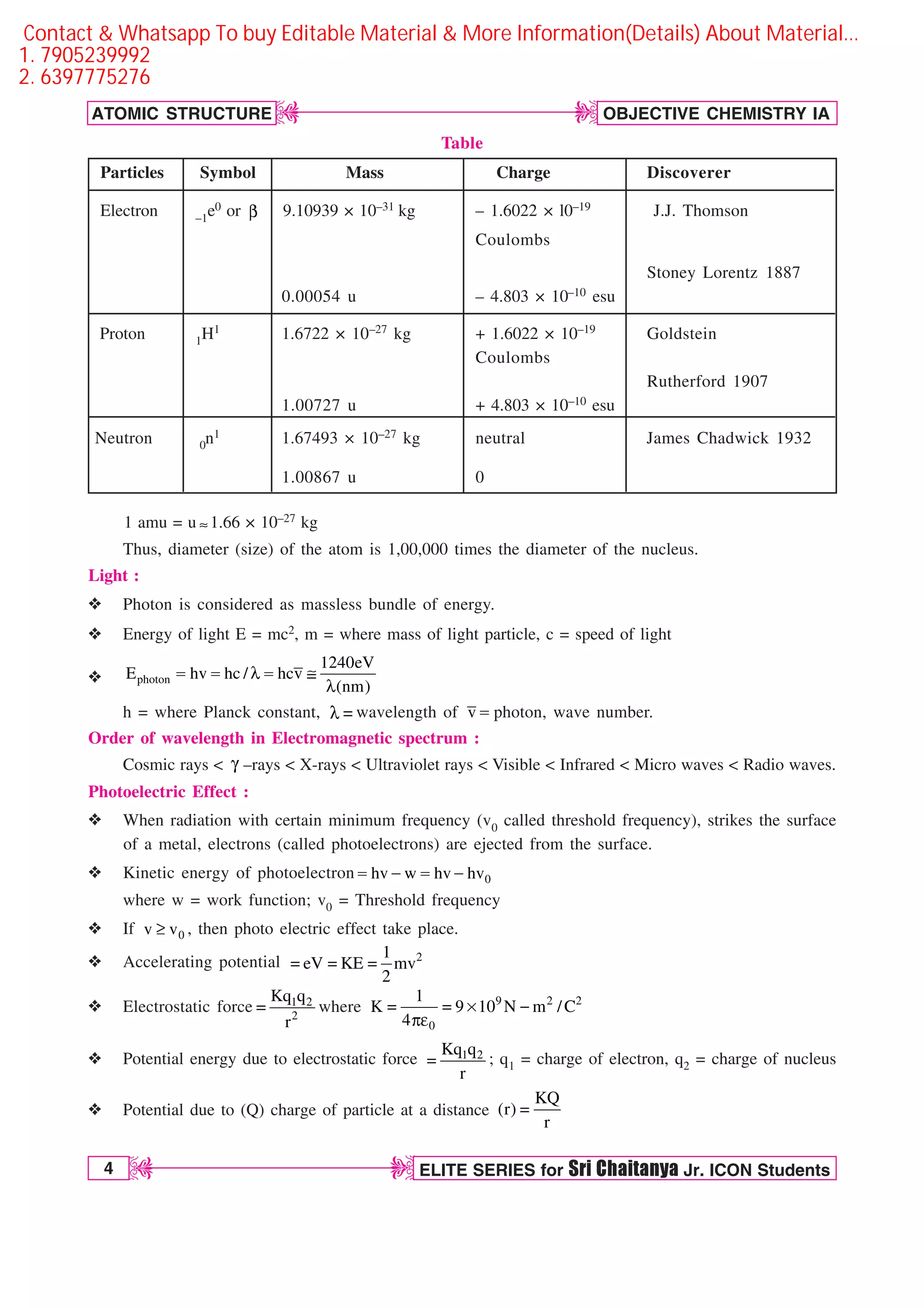
The document discusses atomic structure and provides details about atomic number, mass number, isotopes, and other atomic terms. It describes Rutherford's model of the atom including the discovery of the electron, proton, and neutron as fundamental atomic particles. Bohr's model of the hydrogen atom is explained along with concepts like energy levels, ionization energy, and spectral lines. Other quantum mechanical models like de Broglie's hypothesis, Heisenberg's uncertainty principle, and Schrodinger's wave equation are introduced. Atomic orbitals and the four quantum numbers - principal, azimuthal, magnetic, and spin - are defined.













![18 ELITE SERIES for Sri Chaitanya Jr. ICON Students
OBJECTIVE CHEMISTRY IA
ATOMIC STRUCTURE
D
D
D
D
10. A particle X moving with a certain velocity has a debroglie wave length of 1Å, If particle Y has a
mass of 25% that of X and velocity 75% that of X, debroglies wave length of Y will be
a) 3 Å b) 5.33 Å c) 6.88 Å d) 48 Å
11. Which of the following statement is incorrect ?
a) The third quantum shell can hold a maximum of 18 electrons
b) An electron falling to the same energy level from any higher level always emits the same quantum
of energy
c) The Balmer series of lines is in the visible region of the emission spectrum of hydrogen atom
d) The electron of hydrogen atom in its ground state remains in the first quantum shell
12. The schrodinger wave equation for hydrogen atom is
¥ ´ ¥ ´
:
¦ µ ¦ µ
§ ¶ § ¶
Q
0
3/2
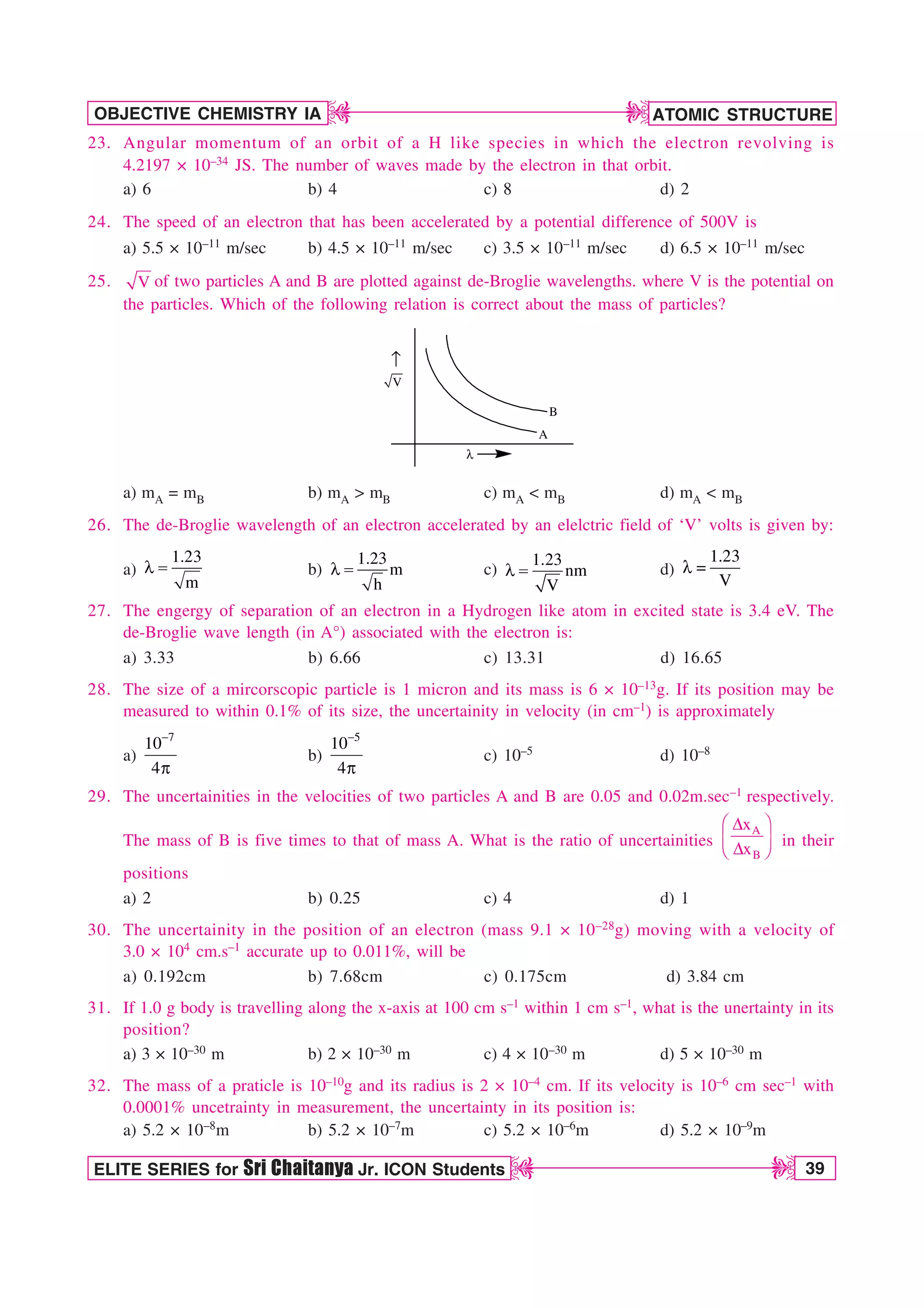
r /a
0
2s
0 0
1 1 r
2 e
a a
4 2
where a0
is Bohr’s radius. If the radial node in 2s be at r0 would be equal to:
a)
0
a
2
b) 2a0 c) 0
2a d)
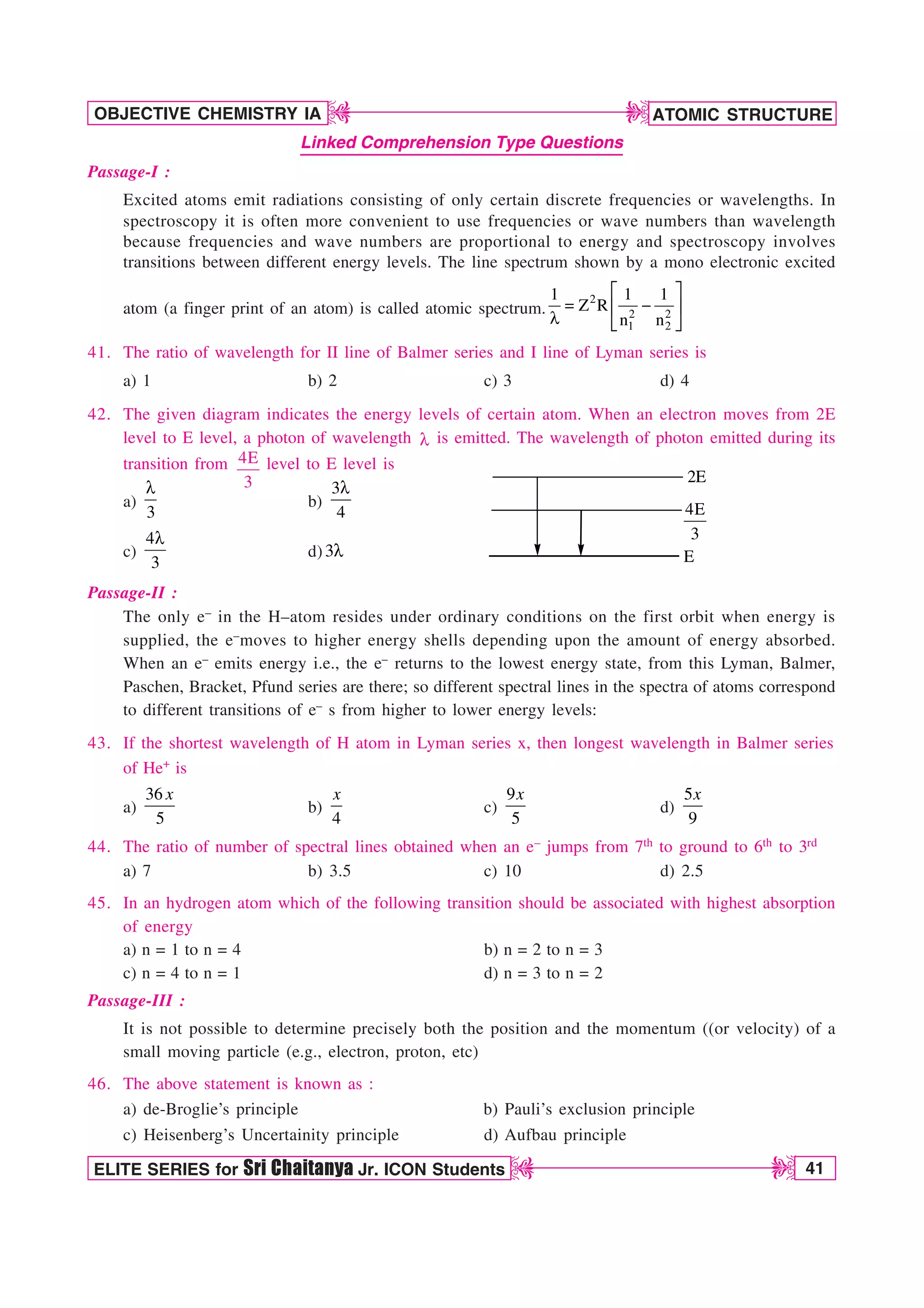
0
a
2
13. The schrodinger wave equation for hydrogen atom is
:(radial)
3 / 2
2 / 2
0
1 z
[( 1)( 8 12)]e
a
16 4
T
¥ ´
T T T
¦ µ
§ ¶
where a0 and z are the constant in which answer can be expressed and
0
2zr
a
T minimum and
maximum position of radial nodes from nucleus are
a)
0 0
a 3a
,
Z Z
b)
0 0
a a
,
2Z Z
c)
0 0
a 3a
,
2Z Z
d)
0 0
a 4a
,
2Z Z
14. For a 3s – orbital
3/ 2
2 / 2
0
1 1
(3s) (6 6 )e ;
a
9 3
T
¥ ´
: T T
¦ µ
§ ¶
where
0
2r.Z
3a
T . What is the maximum
radial distance of node from nucleus?
a) 0
(3 3)a
z
b)
0
a
z
c) 0
(3 3)a
3
2 z
d)
0
2a
z
More than One correct answer Type Questions
15. Which of the following statements is/are correct ?
a) The absorption spectrum is formed due to absorbing radiant energy by the matter in lower energy
states
b) The emission spectrum is formed due to the emission of radiant energy by the excited matter
c) Hydrogen spectrum is an example of line spectrum
d) Li+ ion spectrum is equivalent to H atom spectrum
16. The electron in a hydrogen atom makes a transition n1 mn2 where n1 and n2 are the principal quantum
numbers of the two states. Assume the Bohr model to be valid, if the time period of the electron in
the initial state is eight times that in the final state, then the possible values of n1 and n2 are
a) n1 = 4, n2 = 2 b) n1 = 8, n2 = 2
c) n1 = 8, n2 = 1 d) n1 = 6, n2 = 3](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-16-2048.jpg)


![22 ELITE SERIES for Sri Chaitanya Jr. ICON Students
OBJECTIVE CHEMISTRY IA
ATOMIC STRUCTURE
D
D
D
D
13. Which of the following explains the sequence of filling electrons in different subshells?
1) Hund’s rule 2) Aufbau principle 3) Pauli’s principle 4) All of these
14. Nitrogen atom has 3 unpaired electrons in its ground state. It can be explained by
1) Auf bau principle 2) Pauli’s principle 3) Hund’s rule 4) All of these
15. The electronic configuration of sodium is
1) [Ne]3s2 2) [Ne]3s1 3) [Ar]4s1 4) [Ar]4s2
16. Which of the following configuration may represent the ground state of nitrogen atom?
1) ↓↑ ↓↑ ↑ ↓ ↓ 2) ↓↑ ↓↑ ↑ ↑ ↑ 3) nl nl n n l 4) nl l n ln n
17. Electronic configuration of the element with atomic number 56 and mass number 138 is
1) [Xe]6s2 2) [Kr]5s2 3) [Xe]6s2 6p2 4) [Xe]3d2 5d2
18. In potassium the order of energy levels is
1) 4s 3d 2) 4s 3d 3) 4s 3p 4) 4s = 3d
19. The number of d-electrons retained in Fe2+ (At. no. of Fe = 26) ion is
1) 3 2) 4 3) 5 4) 6
20. Which of the following is the correct electronic configuration of Fe3+ ion ? (Z for Fe = 26)
1) 1s2, 2s2, 2p6, 3s2, 3p6, 3d4 4s1 2) 1s2, 2s2, 2p6, 3s2, 3p6, 3d3 4s2
3) 1s2, 2s2, 2p6, 3s2, 3p6, 3d5 4) 1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s2, 4p3
21. Number of unpaired electrons in the electrmonic configuration 1s22s22p4 is
1) 2 2) 3 3) 4 4) 6
LEVEL-II (ADVANCED)
Straight Objective Type Questions
1. In a multi - electron atom, which of the following orbitals described by the three quantum numbers
will have the same energy in the absence of magnetic and electric field
i) n = 1, l = 0, m = 0 ii) n = 2, l = 0, m = 0 iii) n = 2, l = 1, m = 1
iv) n = 3, l = 2, m = 1 v) n = 3, l = 2, m = 0
a) i and ii b) ii and iii c) iii and iv d) iv and v
2. An electron has magnetic quantum number as ‘–3’. Its principal quantum number is
a) 3 b) 2 c) 1 d) 4
3. Match the following.
LIST-I LIST-II
a) n = 2, l = 1, m = –1 p) 2px or 2py
b) n = 4, l = 2, m = 0 q) 4dz2
c) n = 3, l = 1, m = p1 r) 3px or 3py
d) n = 4, l = 0, m = 0 s) 4s
e) n = 3, l = 2, m = p2 t) 3dx2 – y2 or 3dxy
a) a-q, b-r, c-p, d-s, e-t b) a-t, b-r, c-s, d-p, e-t c) a-p, b-q, c-r, d-s, e-t d) a-s, b-t, c-r, d-s, e-p
K
K](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-20-2048.jpg)
![ATOMIC STRUCTURE
23
OBJECTIVE CHEMISTRY IA
ELITE SERIES for Sri Chaitanya Jr. ICON Students
D
D
D
D
4. The quantum numbers of four electrons (e1 to e4) are given below
n l m s n l m s
e1 3 0 0 +1/2 e2 4 0 1 1/2
e3 3 2 2 –1/2 e4 3 1 –1 1/2
The correct order of decreasing energy of these electrons is:
a) e4 e3 e2 e1 b) e2 e3 e4 e1 c) e3 e2 e4 e1 d) e1 e3 e4 e2
5. Which orbital gives an electron the greatest probability of closer to the nucleus ?
a) 3p b) 3d c) 3s d) equal
6. Which one of the following statements is correct ?
a) 2S orbital is spherical with two nodal planes
b) The de-Borglie wavelength of a particle of mass ‘m’ and velocity ‘v’ is equal to mv/h
c) The principal quantum number (n) indicates the shape of the orbital
d) The electronic configuration of phosphorus is given by [Ne]
2 1 1 1
x y z
3s 3p 3p p
7. The set of quantum numbers ‘n’ and ‘l’ possible for the orbital shown in the radial probability curve are
a) n = 3; l = 2
b) n = 4; l = 1 D
Ao
c) n = 2; l = 0
d) n = 3; l = 3
8. From the following observations predict the type of orbital :
Observation 1: x y plane acts as a nodal plane
Observation 2: The angular function of the orbital intersect the three axis at origin only.
Observation 3: R2(r) vs r curve is obtained for the orbital is
a) 5pz
b) 6dxy
c) 6 dx2–y2
d) 6 dyz
More than One correct answer Type Questions
9. Choose the correct statement.
a) Splitting of spectral lines in magnetic field is due to presence of degenerate orbitals
b) In the presence of electricfield, energy value of Px, Py Pz of same orbit are different
c) Degenerate orbitals differs only in their orientation
d) degenerate orbitals have same shape, but different energy
10. Which of the following statements are not correct ?
a) The ionization energy of a hydrogen-like species in its ground state is equal to the magnitude of
energy of the orbit having n = 1
b) The ionization energy of a hydrogen-like species in its ground state increases in proportion to the
positive charge in its nucleus
c) According to the uncertainty principle, p x h / 4
% % b Q
d) The energy of an electron in an orbital of a multielectron atom depends only on the principal
quantum number n](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-21-2048.jpg)
![24 ELITE SERIES for Sri Chaitanya Jr. ICON Students
OBJECTIVE CHEMISTRY IA
ATOMIC STRUCTURE
D
D
D
D
11. For the energy levels in an atom, which one of the following statement(s) is (are) correct?
a) There are seven principal electron energy levels
b) The second principal energy level can have four sub-energy levels and contain a maximum of
eight electrons
c) The M energy level can have a maximum of 32 electrons
d) The 4s sub-energy level is at a lower energy than the 3d sub-energy level.
12. Which of the following statements are correct ?
a) The angular momentum of an electron due to its spinning is given as
h
s(s 1)
2Q
(where s can
take the value of 1/2)
b) The angular momentum of an electron due to its spinning is given as ms(h/ 2 Q )where ms can
take the value of +1/2.
c) According to Pauli's exclusion principle, no two electrons in an atom can have the same values of
quantum numbers n, l and m
d) The azimuthal quantum number cannot have negative values.
13. The chlorine atom has
a) 6 electrons with l = 0 b) 11 electrons with l = 1
c) 5 orbitals with m = 0 d) 2 orbitals with m = +1
14. Which of the following sets of quantum numbers represents the orbitals of same shape and orientation
in external field.
a) n = 3, l = 2, m = +1, s = +1/2 b) n = 3, l = 2, m = –1, s = –1/2
c) n = 4, l = 2, m = +1, s = –1/2 d) n = 3, l = 2, m = 0, s = +1/2
15. Choose the correct statement(s):
a) The shape of an atomic orbital depends upon azimuthal quantum number
b) The orientation of an atomic orbital depends upon the magnetic quantum number
c) The energy of an electron in an atomic orbital of multi-elelctron atom depends on principal
quantum number only
d) The number of degenerate atomic orbitals of one type depends upon the value of azimuthal
quantum number
16. Select the correct configurations among the following
a) 5 1
Cr(Z 24):[Ar]3d ,4s
b) 10 1
Cu(Z 29):[Ar]3d ,4s
c) 10 0
Pd(Z 46):[Kr]4d ,5s
d) 10 2
Pt(Z 78):[Xe]4d 4s
Linked Comprehension Type Questions
Passage :
The substances which contain species with unpaired electrons in their orbitals behave as paramagnetic
substances. Such substances are weakly attracted by the magnetic field. The paramagnetism is
expressed in terms of magneticmoment The magnetic moment is related to the number of unpaired
electrons according to the following relation : Magnetic moment, n(n 2)
N B.M .
Where ‘n’ = number of unpaired electrons. B.M stands for Bohr magneton, a unit of magnetic
moment.
17. Which of the following has the highest magnetic moment ?
a) Fe+2 b) Mn+2 c) Cr+3 d) V+3](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-22-2048.jpg)






![ATOMIC STRUCTURE
45
OBJECTIVE CHEMISTRY IA
ELITE SERIES for Sri Chaitanya Jr. ICON Students
D
D
D
D
11. When n = 3 and l = 1, the designation given to the orbital is
1) 4s 2) 4p 3) 3s 4) 3p
12. Which of the following designation is impossible?
1) 4f 2) 5g 3) 2d 4) 6p
13. The correct valence electronic configuration for Cu(Z = 29) is
1) 3d9 4s2 2) 3d10 4s1 3) 3d10 4s2 4) 3d8 4s2
14. Which one of the following pairs of ions have the same electronic configuration
1) Cr3+, Fe3+ 2) Fe3+, Mn2+ 3) Fe3+, Co3+ 4) Sc3+, Cr3+
15. The (n + l) value for 4f-sub shell is
1) 4 2) 5 3) 6 4) 7
16. The energy of the electron in the hydrogen atom depends on
1) The principal quantum number only
2) All the quantum numbers
3) The Azimuthal quantum number
4) The principal and azimuthal quantum numbers
17. After 3d-sub level is completely filled the differentiating electron enters into ____ sub level.
1) 4s 2) 4p 3) 4f 4) 5s
18. The correct ground state electronic configuration of chromium atom is
1) [Ar] 3d5 4s1 2) [Ar] 3d4 4s2 3) [Ar] 3d6 4) [Ar] 3d5 4s2
19. Consider the following pairs of ions
i) Sc+3 and Ti+4 ii) Mn+2 and Fe+2 iii) Fe+2 and Co+3 iv) Cu+ and Zn+2
Among these pairs of ions, isoelectronic pairs would include
1) ii, iii and iv 2) i, iii and iv 3) i, ii and iv 4) i, ii and iii
LEVEL-II (ADVANCED)
Straight Objective Type Questions
1. The number of sub levles in the quantum level n = 3 is
a) 1 b) 2 c) 3 d) 4
2. The number of different spatial arrangements for the orbital with l = 2 is
a) 1 b) 3 c) 5 d) 7
3. An electron in an atom has m = –2 value. Then
I) its ‘n’ value should be greater than 2 II) its ‘s’ value should be + 1/2
III) its ‘l’ value should be 2 IV) its ‘l’ value should be greater than 1
a) I and II are correct b) II and III are correct
c) III and IV are correct d) I and IV are correct
4. How many electrons in an atom with atomic number 105 can have (n + l) = 8?
a) 30 b) 15 c) 17 d) 16
K
K](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-43-2048.jpg)
![ATOMIC STRUCTURE
47
OBJECTIVE CHEMISTRY IA
ELITE SERIES for Sri Chaitanya Jr. ICON Students
D
D
D
D
14. There is no difference between a 2p and a 3p orbital regarding
a) Value of n b) Size c) Energy d) Shape
15. The probability of finding electron in XY plane for PZ– orbital is
a) 100% b) 50% c) 99.9% d) 0%
16. How many electrons maximum can have n + l = 4 in an atom.
a) 8 b) 2 c) 6 d) 18
17. In which of the following Aufbau principle is violated ?
a) n
l n
l l l
2s 2p
b) l n
l l l
2s 2p
c) n
l l l l
2s 2p
d) n
l n
l l l
2s 2s
n
18. Which of the following electronic configuration is not possible?
a) 1s2 2s2 2p6 b) 1s2 2s2 2p7 c) 1s2 2s2 d) 1s2 2s2 2p5
19. The configuration 1s2 2s2 2p5 3s1 shows
a) Ground state of fluorine b) Excited state of fluorine
c) Exited state of neon atom d) Excited state of argon
20. Which of the following has maximum unpaired d-electrons?
a) Zn+ b) Fe2+ c) Ni3+ d) Cu+
21. The number of electrons in the ground state of atom (z = 24) with the quantum numbers l = 1 and
l = 2 are, respectively
a) 12, 4 b) 12, 5 c) 16, 4 d) 16, 5
22. For H-atom, the energy required for the removal of electron from various sub-shells is given as
under:
The order of the energies would be :
a) E1 E2 E3 b) E3 E2 E1 c) E1 = E2 = E3 d) E1 E3 E2
23. Among the following representations of excited states of atoms which is impossible ?
a) 1s1 2s1 b) 3s2 3p3 4s1 c) 1s2 2s2 2p4 3s2 d) [Ne] 3s2 3p6 3d2 4s3
24. Consider the following six electronic configurations (remaining inner orbitals are completely filled)
and mark the incorrect option.
I) ↑ ↑ ↑ ↑ II) ↑ ↑ ↑
↓
III) ↑ ↑ ↑ ↑
↓ ↑ IV) ↑ ↑ ↑ ↑ ↑ ↑
V) ↑ ↑ ↑ ↑↑ VI) ↑ ↑ ↑
↓
a) Stability order : IV II III b) Order of spin multiplicity : IV III = I II
c) V does not violate all the three rules of electronic configuration
d) If IV represents A+ when kept near a magnet, acts as diamagnetic substance.](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-45-2048.jpg)

![ATOMIC STRUCTURE
49
OBJECTIVE CHEMISTRY IA
ELITE SERIES for Sri Chaitanya Jr. ICON Students
D
D
D
D
33. Choose the correct statement(s):
a) for a particular orbital in hydrogen atom, the wave function may have negative value
b) Radial probability distribution function may have zero value but can never have negative value
c) 2 2
x y
3d
orbital has two angular nodes and one radial node
d) yz and xz planes are nodal planes for dxy orbital
34. Which of the following statements are correct for an electron that has n = 4 and m = –2?
a) The electron may be in a d-orbital
b) The electron is in the fourth principal electronic shell
c) The electron may be in a p-orbital
d) The electron must have the spin quantum number = +1/2.
35. Which of the following statements are correct ?
a) The number of orbitals for a given value of l is equal to 2l + 1
b) The number of orbitals for a given value of n is equal to n2
c) An atom having unpaired electrons is diamagnetic is nature
d) The half-filled and fully-filled electronic configurations are less stable than the other configura-
tion having the same number of electrons.
36. Select the correct statement(s):
a) Radial distribution function indicates that there is a higher probability of finding the 3s electron
close to the nucleus than in case of 3p and 3d orbitals
b) Energy of 3s orbital is less than for the 3p and 3d orbitals
c) At the node, the value of the radial function changes from positive to negative
d) The radial function depends upon the quantum numbers n and l
37. A neutral atom has 2K, 8L and 5M electrons, choose correct one
a) Atomic number is 15
b) Total number of p-electrons = 9
c) Valency of element is 2
d) It is a representative element.
38. Which of the following are correct ?
a) All s-orbitals have the same orbital angular momentum.
b) The angular momentum of the electron in 4th orbit is 2h/ Q
c) The magnetic moment of Cu2+ ion = 1.732 B.M.
d) All d-orbitals have two nodal planes.
39. The configuration [Ar]3d54s1 in the first excited states exists for
a) Fe2+ b) Co3+ c) Mn+ d) Cr
40. The following electronic configuration violates
↑ ↑ ↑ ↑ ↑
↑
↑ ↑ ↑↑
a) Hund’s rule b) Pauli’s principle
c) Aufbau principle d) Heisenberg’s principle](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-47-2048.jpg)
![50 ELITE SERIES for Sri Chaitanya Jr. ICON Students
OBJECTIVE CHEMISTRY IA
ATOMIC STRUCTURE
D
D
D
D
41. Ground state electronic configuration of p atom can be represented as
a)
3s 3p
↑n ↑ ↑ ↑
[Ne] b)
3s 3p
↑n ↑ ↑
[Ne] n c)
3s 3p
↑n ↑
[Ne] n n d)
3s 3p
↑n
[Ne] n n
n
42. Which of the following statement is/are wrong?
a) If the value of l = 0, the electron distribution is spherical
b) The shape of the orbital is given by magnetic quantum no.
c) Angular moment of 1s, 2s, 3s electrons are equal
d) In an atom, all electrons travel with the same velocity
43. Gaseous state electronic configuration of Nitrogen atom can be represented as:
a) ln ln l l l b) ln ln l n l c) ln ln l n n d) ln ln n n n
44. The electronic configuration of an element is 1s2 2s2 2p6 3s2 3p6 3d5 4s1. This represents its:
a) excited state b) ground state c) cationic form d) anionic form
Linked Comprehension Type Questions
Passage-I :
It is tempting to think that all possible transitions are permissible, and that an atomic spectrum arises
from the transition of the electron from any initial orbital to any other orbital. However, this is not so,
because a photon has an intrinsic spin angular momentum of
h
2
2Q
corresponding to S = 1 although
it has no charge and no rest mass. On the other hand, an electron has got two types of angular
momentum :
Orbital angular momentum,
h
L ( 1)
2
Q
l l and spin angular momentum, arising from orbital motion
and spin motion of electron respectively. The change in angular momentum of the electron during
any electronic transition mush compensate for the angular momentum carries away by the photon.
to satisfy this condition the difference between the aziuthal quantum numbers of the orbital within
which transition takes place must differ by one. Thus, an electron in a d-orbital (1 = 2) cannot make
a transition into an s = orbital (I = 0) because the photon cannot carry away enough angular
momentum. An electron as is well known, possess four quantum numbers n, I, m and s. Out of
these four I determines the magnitude of orbital angular momentum (mentioned above) while
m determines its z-components as
h
m
2
¥ ´
¦ µ
§ ¶
Q
The permissible values of only integers right from
–1 to + l. While those for I are also integers starting from 0 to (n – 1). The values of I denotes the sub-
shell. For I = 0, 1, 2, 3, 4,….. the sub-shells are denoted by the symbols s, p, d, f, g, …. respectively
45. The maximum orbital angular momentum of an electron with n = 5 is
a)
h
6
2Q
b)
h
12
2Q
c)
h
42
2Q
d)
h
20
2Q
46. The orbital angular momentum of an electron in p-orbital makes an angle of 45° from Z-axis.
Hence Z-component of orbital angular momentum of electron is :
a)
h
Q
b)
h
2
¥ ´
¦ µ
§ ¶
Q
c)
h
Q
d)
h
2
¥ ´
¦ µ
§ ¶
Q
47. The spin-only magnetic moment of free ion is 8 B.M. The spin angular momentum of electron
will be
a)
h
2
2Q
b)
h
8
2Q
c)
h
6
2Q
d)
3 h
4 2Q](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-48-2048.jpg)

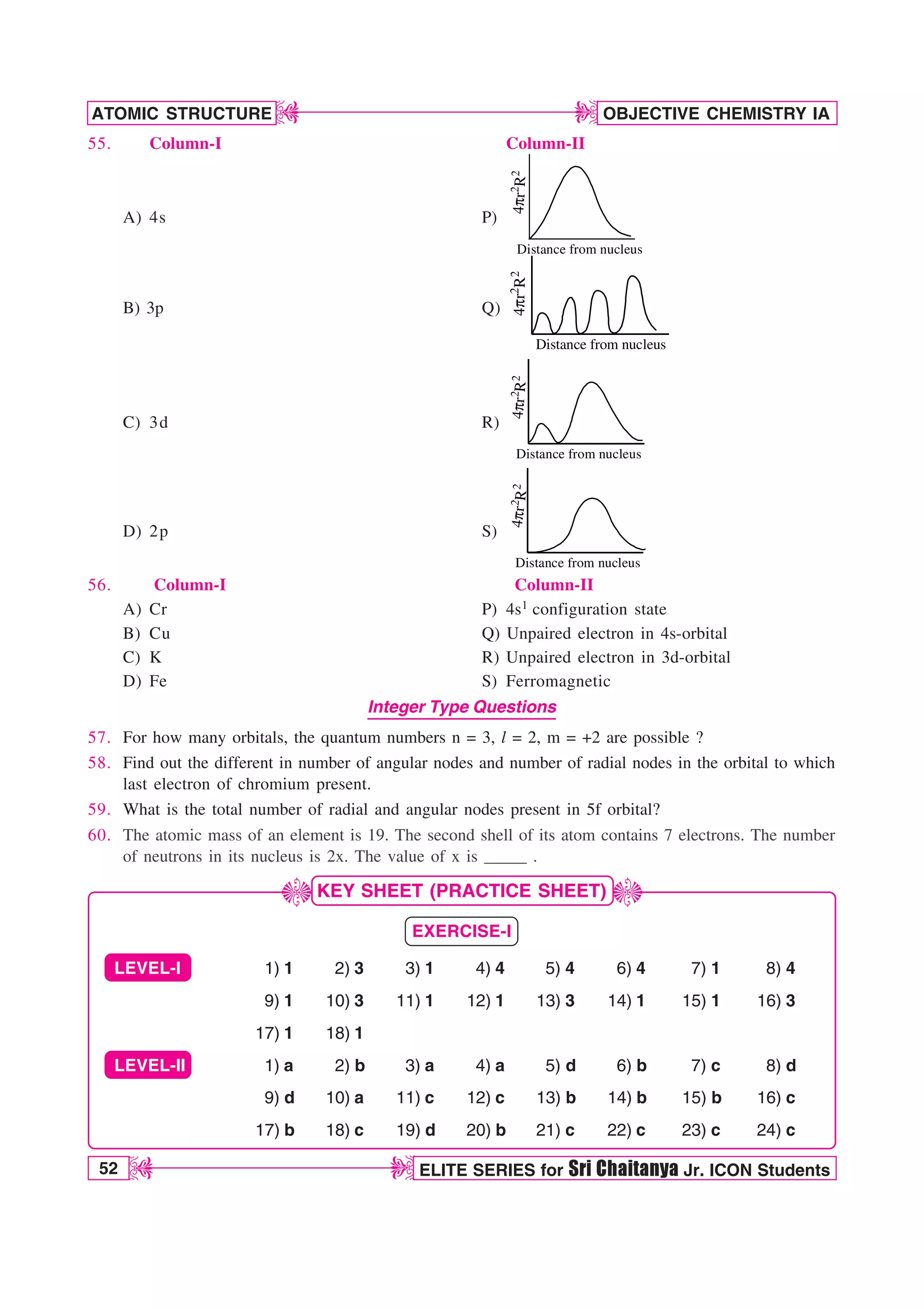
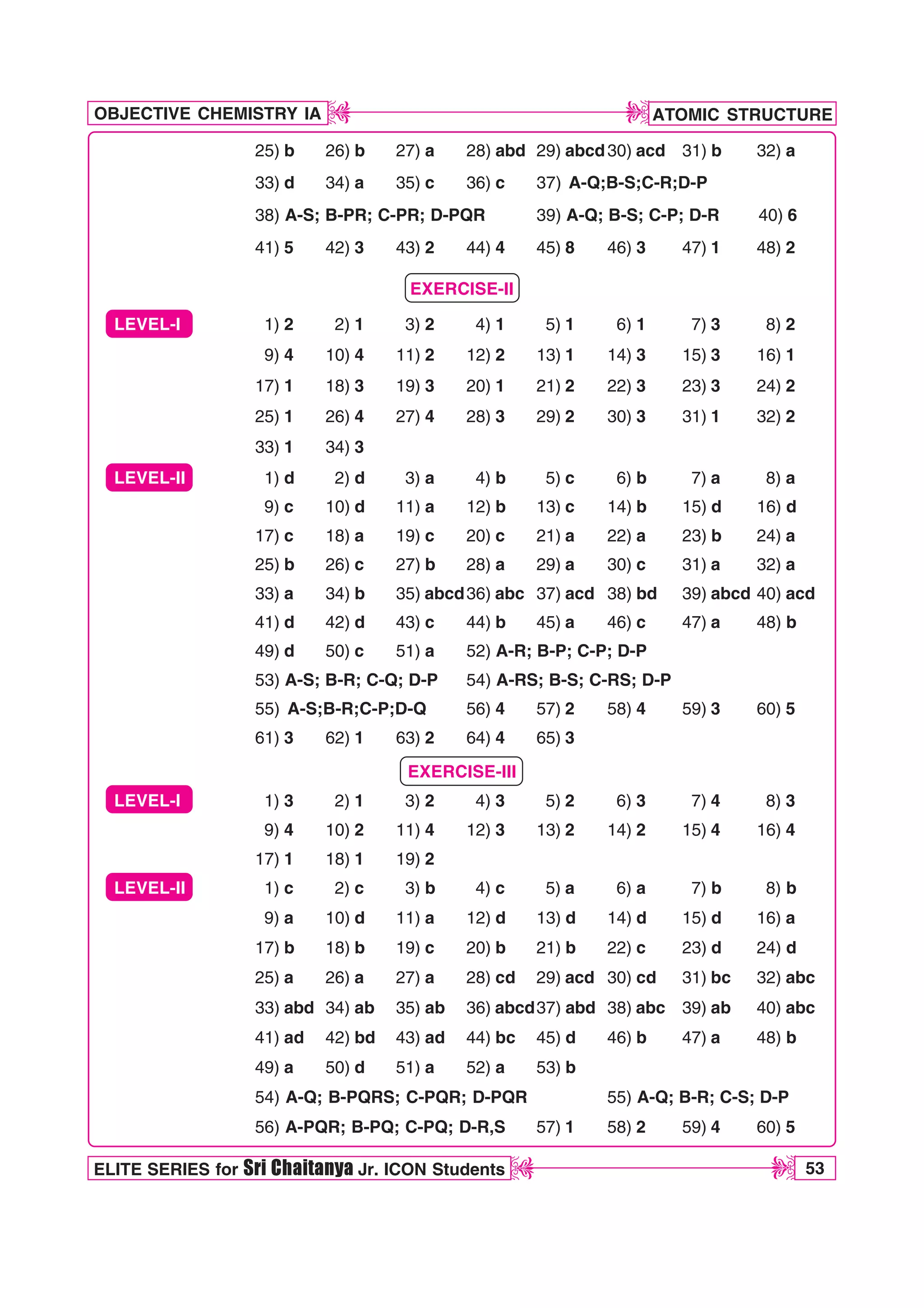

![ATOMIC STRUCTURE
55
OBJECTIVE CHEMISTRY IA
ELITE SERIES for Sri Chaitanya Jr. ICON Students
D
D
D
D
12. If magnetic quantum number of a given electron is represented –3, then what will be its principal
quantum number ?
1) 2 2) 3 3) 4 4) 1
13. The configuration of 1s2 2s2 2p6 3s1 3p1 shows
1) Ground state of fluorine atom 2) Excited state of fluorine atom
3) Excited state of magnesium atom 4) Excited state of oxygen atom
14. The incorrect electronic arrangement is
1) 2, 8, 13, 1 2) 2, 8, 12, 2 3) 2, 8, 8, 1 4) 2, 8, 8, 2
15. Given this set of quantum numbers for a multi-electron atom 2, 0, 0, and –1/2. The next higher
allowed set of n and l for this atom in its ground state
1) n = 2, l = 0 2) n = 2, l = 1 3) n = 3, l = 0 4) n = 3, l = 1
16. Which one of the following pairs of atoms/ions have identical ground state configurations?
1) Li+ and He+ 2) Cl– and Ar 3) Na and K 4) F+ and Ne
17. The atomic number of an element ‘M’ is 26. How many electrons are present in the M-shell of the
element in its M3+ state ?
1) 11 2) 15 3) 14 3) 13
18. Consider the ground state of Cr atom (Z = 24). The numbers of electrons with the azimuthal quantum
numbers, l = 1 and 2 are, respectively
1) 12 and 4 2) 16 and 5 3) 16 and 4 4) 12 and 5
19. Which is the first element to have 4d-electron in its electronic configurations
1) Ca 2) Sc 3) Y 4) La
20. In the ground state, an element has 13 electrons in its “M - shell”. The element is
1) Copper 2) Chromium 3) Nickel 4) Iron
21. (A) : The electronic configuration of Cr is [Ar]3d54s1 but not [Ar]3d44s2.
(R) : Lowering energy with configuration [Ar]3d54s1 is more than that with configuration [Ar]3d44s2.
1) Both (A) and (R) are true and (R) is the correct explanation of (A)
2) Both (A) and (R) are true and (R) is not the correct explanation of (A)
3) (A) is true but (R) is false 4) (A) is false but (R) is true
22. The electrons identified by quantum numbers n and l are
I) n = 4, l = 1 II) n = 4, l = 0 III) n = 3, l = 2 IV) n = 3, l = 1
can be placed in order of increasing energy as
1) IV II III I 2) II IV I III 3) I III II IV 4) III I IV II
23. Which of the following statement(s) is/are consistent with the Bohr theory of the atom (and no
others) ?
A) An electron can remain in a praticular orbit as long as it continuously absorbs radiation of a
definite frequency.
B) The lowest energy orbits are those closest to the nucleus.
C) All electrons can jump from the K shell to the M shell by emitting ratiation of a definite
frequency.
1) A,B,C 2) B Only 3) C Only 4) A,B](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-53-2048.jpg)

![ATOMIC STRUCTURE
57
OBJECTIVE CHEMISTRY IA
ELITE SERIES for Sri Chaitanya Jr. ICON Students
D
D
D
D
35. The maximum number of electrons in a sub-shell is given by the expression.
1) (l + 2) 2) (2l + 2) 3) (4l + 2) 4) (l + 1)
36. The magnetic quantum number, m for the outermost electron in the sodium atom is
1) 1 2) 0 3) 2 4) –1
37. For the configuration 1s22s1, the quantum numbers for the outermost electron are
1) 2,1,0, –1/2 2) 2,0,0,+1/2 3) 2,1,0,+1/2 4) 2,0,1,+1/2
38. The maximum number of electrons that a p-orbital can accomodate is
1) 6 2) 2 3) 10 4) 14
39. The number of orbitals in the quantum level n = 4 is
1) 4 2) 9 3) 16 4) 18
40. The quantum number which is equal for all the d-electrons in an atom is
1) l 2) m 3) s 4) n
41. Correct set of four quantum numbers for the valence electron of Rubidium (Z = 37) is
1) 5, 0, 0, +1/2 2) 5, 1, 0, +1/2 3) 5, 1, 1, +1/2 4) 6, 0, 0, +1/2
42. n, l and m values of the 2pz orbital are
1) 3,2,1 2) 2,1,0 3) 1,2,0 4) 2,0,1
43. The azimuthal quantum number for the last electron in sodium atom is
1) 1 2) 2 3) 0 4) 3
44. Which of the following electronic cofiguration corresponds to an inert gas ?
1) 1s22s22p5 2) 1s22s22p6 3) 1s22s22p63s1 4) 1s22s2
45. The reason for chromium to have [Ar]3d54s1 configuration instead of [Ar]3d44s2 is
1) Pauli’s exclusion principle 2) Aufbau principle
3) more exchange energy 4) Heisenberg’s principle
46. Which of the following configuration is not possible?
1) 2p2 2) 3f7 3) 3d5 4) 4p6
47. Which of the following ions is not isoelectronic with O2–
1) N3– 2) F– 3) Ti+ 4) Na+
48. Number of valence electrons in carbon is
1) 3 2) 1 3) 4 4) 0
49. The orbital diagram in which both the Pauli’s exclusing principle and Hund’s rule are violated, is:
1) ↓ ↓ ↓
↑↓ 2) ↑↓ ↑↓ ↑↓ ↑
3) ↑↓ ↑↓ ↑↓ 4) ↑↓ ↑ ↑↓
↑
50. The ratio of magnetic moments of Fe (III) and Co (II) is:
1) 5 : 7 2) 35 : 15 3) 7 : 3 4) 24 : 15](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-55-2048.jpg)



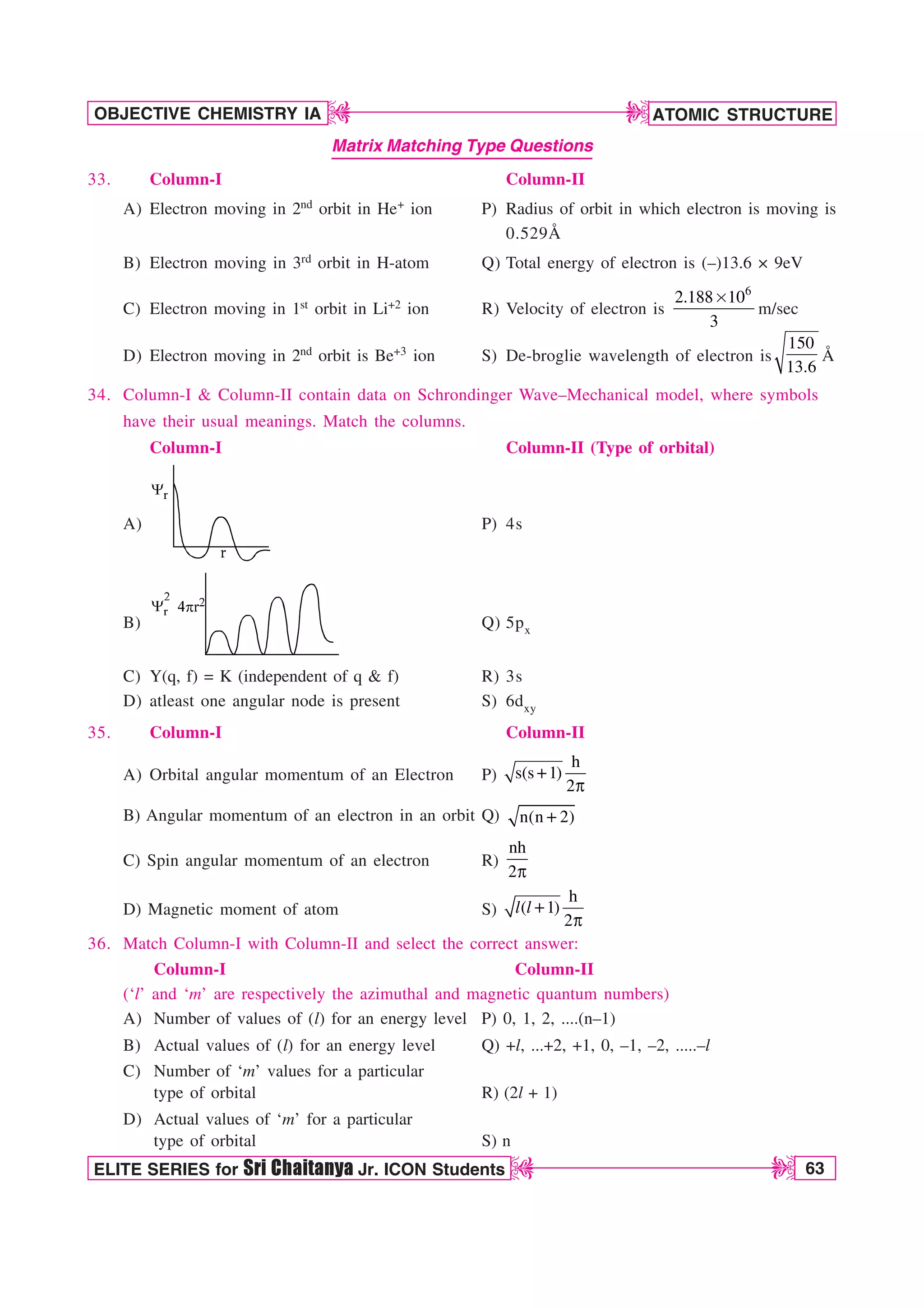

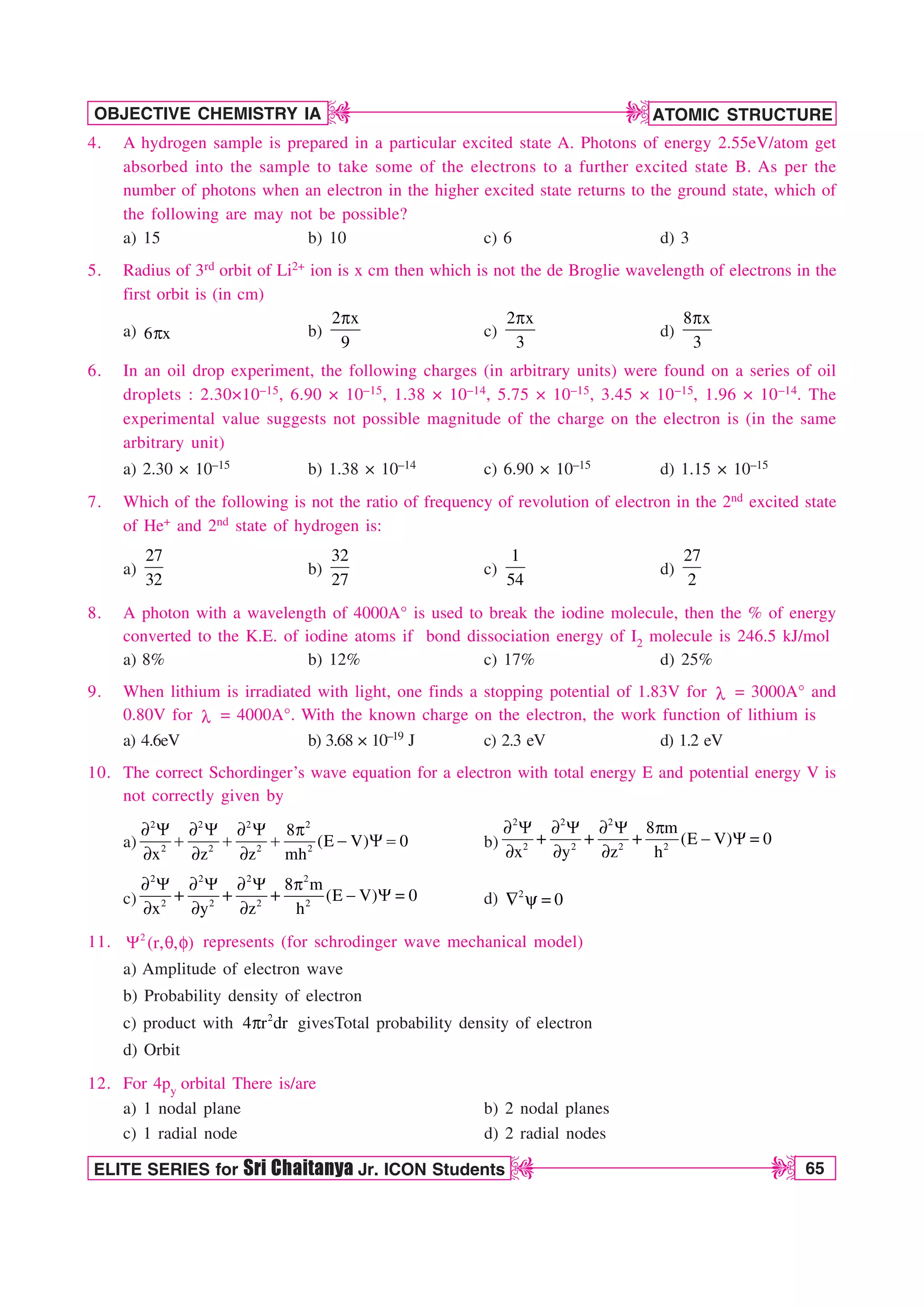
![66 ELITE SERIES for Sri Chaitanya Jr. ICON Students
OBJECTIVE CHEMISTRY IA
ATOMIC STRUCTURE
D
D
D
D
13. Select the correct statement(s) regarding 3pyorbital
a) Total no. of nodes are 2
b) Number of maximuma in the curve 2 2
4 r R
Q vs r are two
c) Quantum no. n, l and m for orbital may be 3,1,–1 respectively
d) The magnetic quantum number may have a positive value
14. Select the correct statement(s):
a) Radial function [R(r)] a part of wave function is dependent on quantum number n only
b) angular function depends only on the direction, and is dependent to the distance from the nucleus
c) 2
(r, , )
: R G is the probability density of finding the electron at a particular point in space
d) Radial distribution function 2
(4 r )
Q gives the probability of the electron being present at a
distance r from the nucleus
15. For which of the following orbital, the radial as well as number of nodal planes have all the same
value ?
a) 3px b) 4py c) 3s d) 5d xy
16. A proton and an B -particle are accelerated through the same potential difference. The incorrect
ratio of the de-Broglie wave length of proton and B -particle is
a) 2 b)
1
2
c) 2 2 d) 2
17. Incorrect Wave number of the second line of Paschen series for H is (R = 109700 cm–1)
a) 18750 Å b) 3452 Å c) 7801 Å d) 1542656 Å
18. Photoelectric emission is observed from a surface for frequencies n1 and n2 of the incident radiation
(n1 n2). If the maximum K.E. of the photoelectrons in two cases are in ratio 1 : K, then the incorrect
expression for threshold frequency is
a)
2 1
K 1
V V
b)
1 2
K
K 1
V V
c)
2 1
K
K 1
V V
d)
2 1
K
V V
19. A particle A moving with a certain velocity has a de-Broglie wavelength of 1 Å. If particle B has
mass 25% of that A and velocity 75% of that of A, the l of B will not be
a) 1 Å b) 5.3 Å c) 0.2 Å d) 3 Å
20. The radius of first Bohr orbit is x, then de-Broglie wavelength of electron in 3rd orbit is not
a) 2 x
Q b) 6 x
Q c) 9 x
Q d) x /3
Q
21. If E1, E2 and E3 represent respectively the kinetic energies of an electron, an alpha particle and a
proton respectively each having same deBroblie wavelength then which is incorrect relation?
a) E1 E3 E2 b) E2 E3 E1
c) E1 E2 E3 d) E1 = E2 = E3
22. Electronic transition in He ion takes from n2 to n1 shell such that :
2n2 + 3n1 = 18 ; 2n2 – 3n1 = 6
What will be the total number of photons emitted when electrons transit to n1 shell ?
a) 21 b) 15 c) 20 d) 10](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-64-2048.jpg)

![68 ELITE SERIES for Sri Chaitanya Jr. ICON Students
OBJECTIVE CHEMISTRY IA
ATOMIC STRUCTURE
D
D
D
D
31.
3
n =
2
n =
1
n =
1
2
3
For above transitions in hydrogen like atoms, select the correct relation(s).
a) 3 1 2
v v v
b)
1 2
3
1 2
v v
v
v v
c) 3 1 2
M M M d) 1 2
3
1 2
M M
M
M M
32. If the electron of the hydrogen atom is replaced by another particle of same charge but of the double
mass, then :
a) radii of different shells will increase
b) energy gap between two levels will become double
c) ionization energy of the atom will be double
d) speed of new particle in a shell will be lesser than the speed of electron in the same shell
33. The radial distribution function [P(r)] is used to determine the most probable radius, which is used to
find the electron in a given orgital
dP(r)
dr
for 1s– orbital of hydrogen like atom having atomic num-
ber Z, is
0
3 2
2Zr /a
3
0
0
dP 4Z 2Zr
2r e
dr a
a
¥ ´
¦ µ
§ ¶
: Then which is the following statements is/are correct ?
a) At the point of maximum value of radial distribution function
dP(r)
0
dr
; one anti-node is present
b) Most probable radius of Li2+ is
0
a
3
pm
c) Most probable radius of He+ is
0
a
2
d) Most probable radius of He+ is
0
a
2
Linked Comprehension Type Questions
Passage :
When electron jumps from higher orbit to lower orbit, then energy is radiated in the form of electro-
magnetic radiation and these radiations are used to record the emission spectrum
Energy of electron may be calculated as
2 2 4
e
2 2
2 m Z e
E
n h
Q
where, me = rest mass of electron 2 1
2
n n 2 2
1 2
1 1
E (E E ) 13.6 Z eV
n n
¨ ·
% s s
© ¸
ª ¹
per atom
This equation was also used by Rydberg to calculate the wave number of a particular line in the
spectrum.
2 1
H 2 2
1 2
1 1 1
R Z m
n n
¨ ·
V
© ¸
M ª ¹](https://image.slidesharecdn.com/atomicstructuresrichaitanya-230620031320-b36337c4/75/Atomic-Structure-sri-chaitanya-pdf-66-2048.jpg)