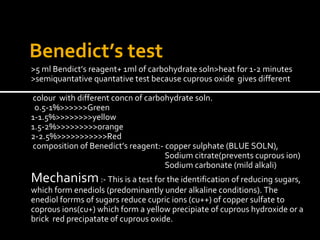
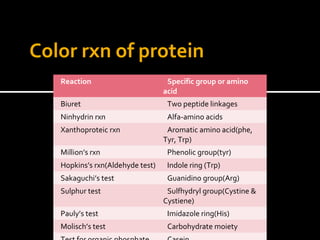
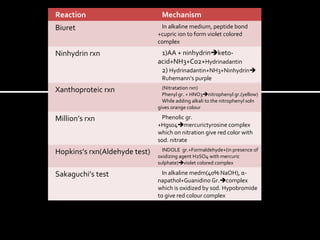
This document summarizes several laboratory tests used to identify carbohydrates and proteins. For carbohydrates, it describes tests such as Molisch's test, iodine test, Benedict's test, Barfoed's test, Seliwanoff's test, and osazone test. It explains the procedures and mechanisms for how each test identifies different types of carbohydrates. For proteins, it lists common color reactions used to detect specific amino acids or protein components, such as the biuret test, ninhydrin test, xanthoproteic test, and others. It provides the reactions and mechanisms for how these color tests can identify proteins and their constituents.