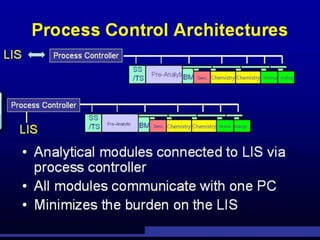
The document discusses the evolution and significance of automation in clinical chemistry, highlighting its ability to increase efficiency in laboratory tests by minimizing the human involvement required. It covers the historical development of various automated systems, such as continuous flow and discrete analyzers, and outlines the technologies involved in specimen processing, identification, and analysis. Key benefits of automation include improved reliability, accuracy, and cost-effectiveness in laboratory operations.