This document discusses pharmaceutical impurities. It defines impurity as unwanted foreign particles other than the active drug. Impurities can come from raw materials, reagents, manufacturing processes, storage conditions, or deliberate adulteration. The types and amounts of impurities depend on factors like purity of starting materials and purification methods. Limit tests are used to detect and limit specific impurities like chlorides, sulphates, and iron according to pharmacopeia limits. The tests use reactions like precipitation or color changes to compare a sample to a standard of a known impurity level. Maintaining low impurity levels is important for safety, efficacy, and stability of pharmaceutical products.
























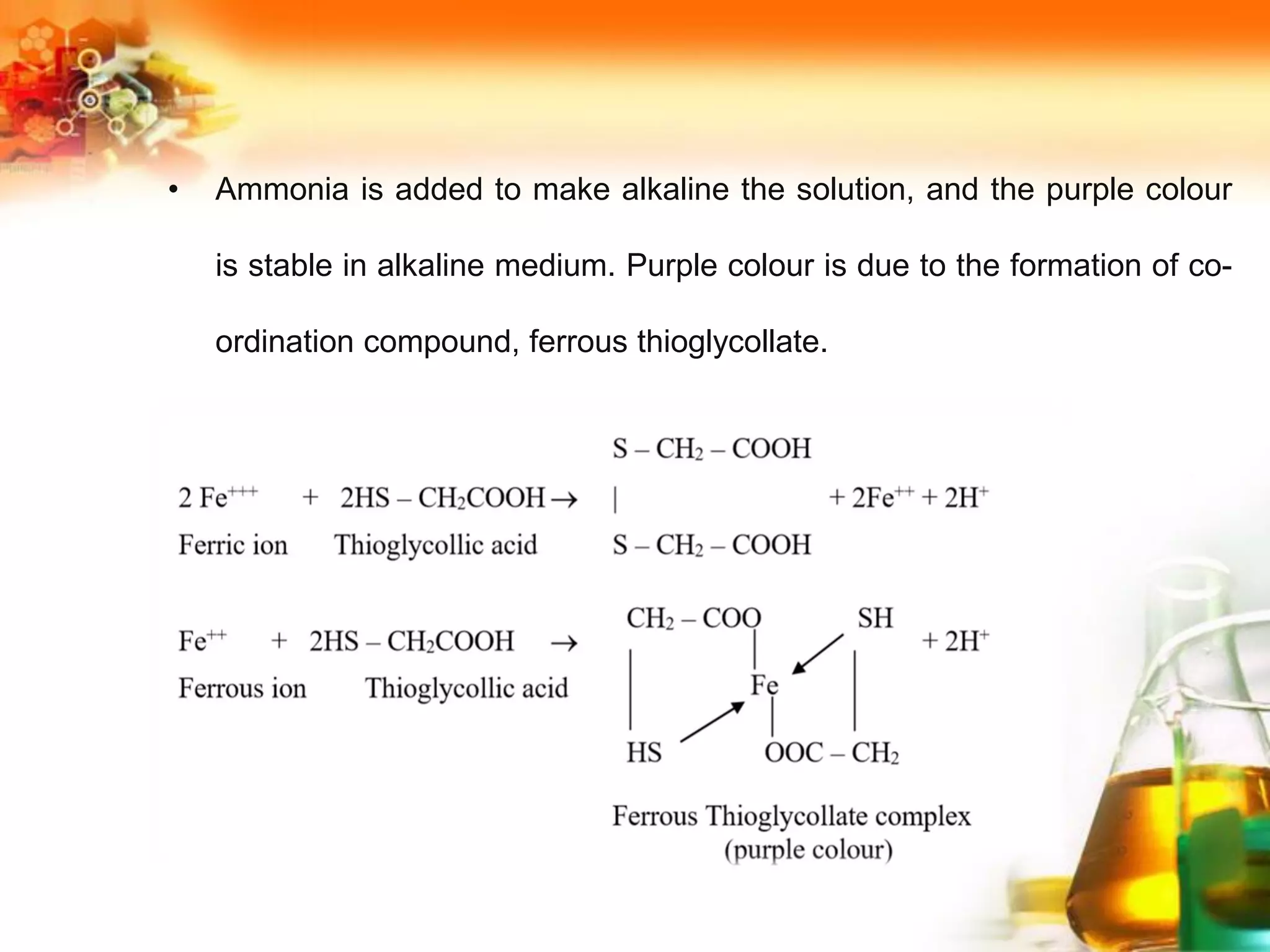





![Reactions Are:
Zn + 2HCl → ZnCl2 + 2 [H]+
H3ASO4 + 2 [H] → H3ASO3 + H2O
H3ASO3 +6 [H] → ASH3 + 3H2O
2ASH3 + HgCl2 → Hg (ASH3)2 + 2HCl](https://image.slidesharecdn.com/sourcesofimpurities-210726103854/75/Sources-of-impurities-33-2048.jpg)


