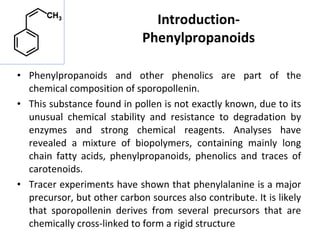
The document discusses various phenylpropanoids and related compounds that are derived from plants, including their chemical structures, biological sources, constituents, and some uses. It provides details on specific phenylpropanoid-containing plants like podophyllum, psoralea, ammi majus, phyllanthus, and male fern, outlining their constituents like lignans, furanocoumarins, polyketides, and resins. The document also gives background on phenolic compounds, monomeric and dimeric derivatives, lignans, lignin, and their roles in plant biology and chemistry.