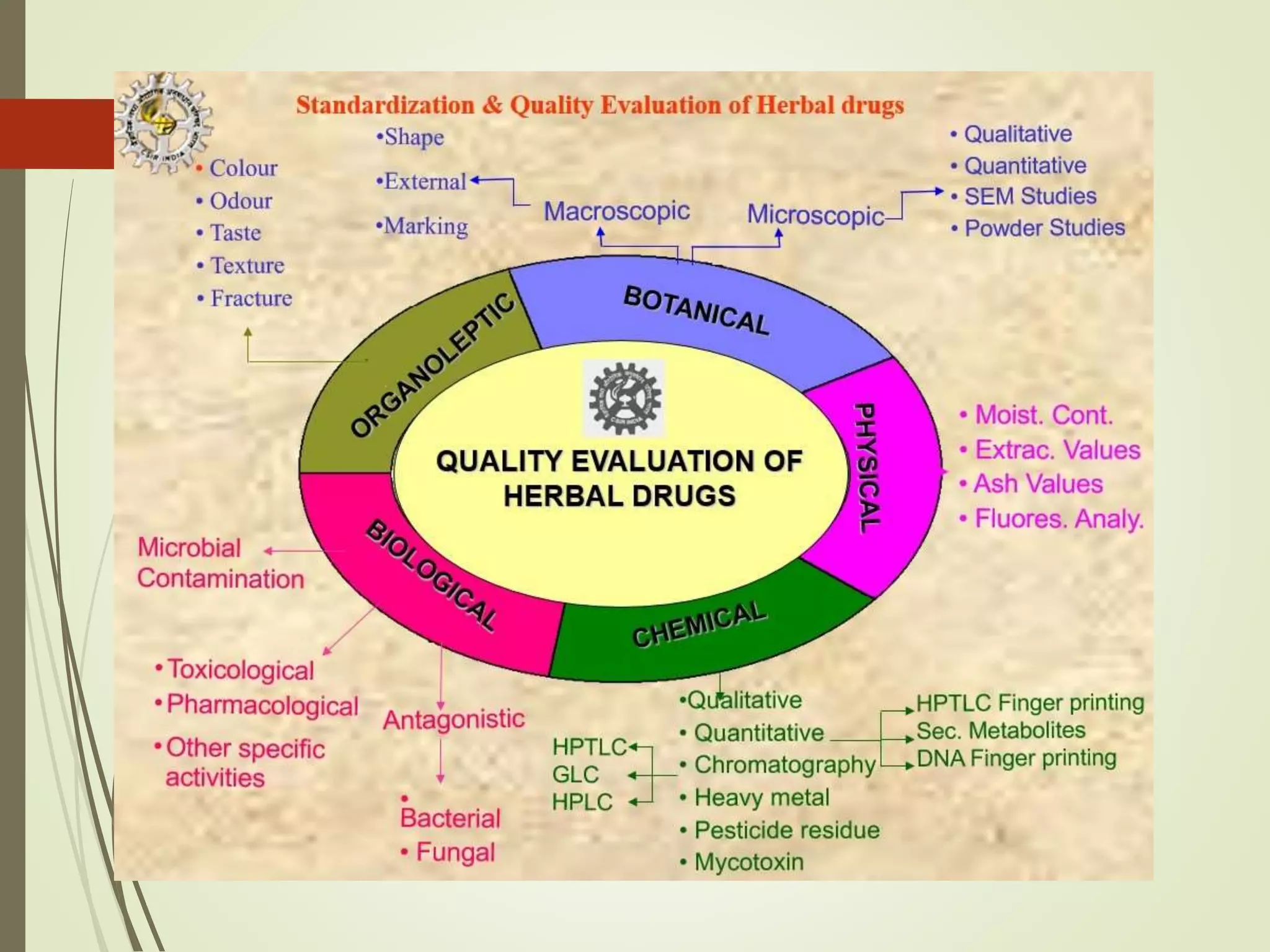
The document discusses regulations for herbal drugs and quality standards. It outlines World Health Organization guidelines for authentication, contaminants testing, and other quality control of herbal drugs. It then describes regulations for herbal drugs in India, the United States, Australia, Canada, and the European Union. It also discusses Schedule T of the Indian Drugs and Cosmetics Act, which lays out good manufacturing practices, and potential interactions between herbal medicines and conventional drugs.