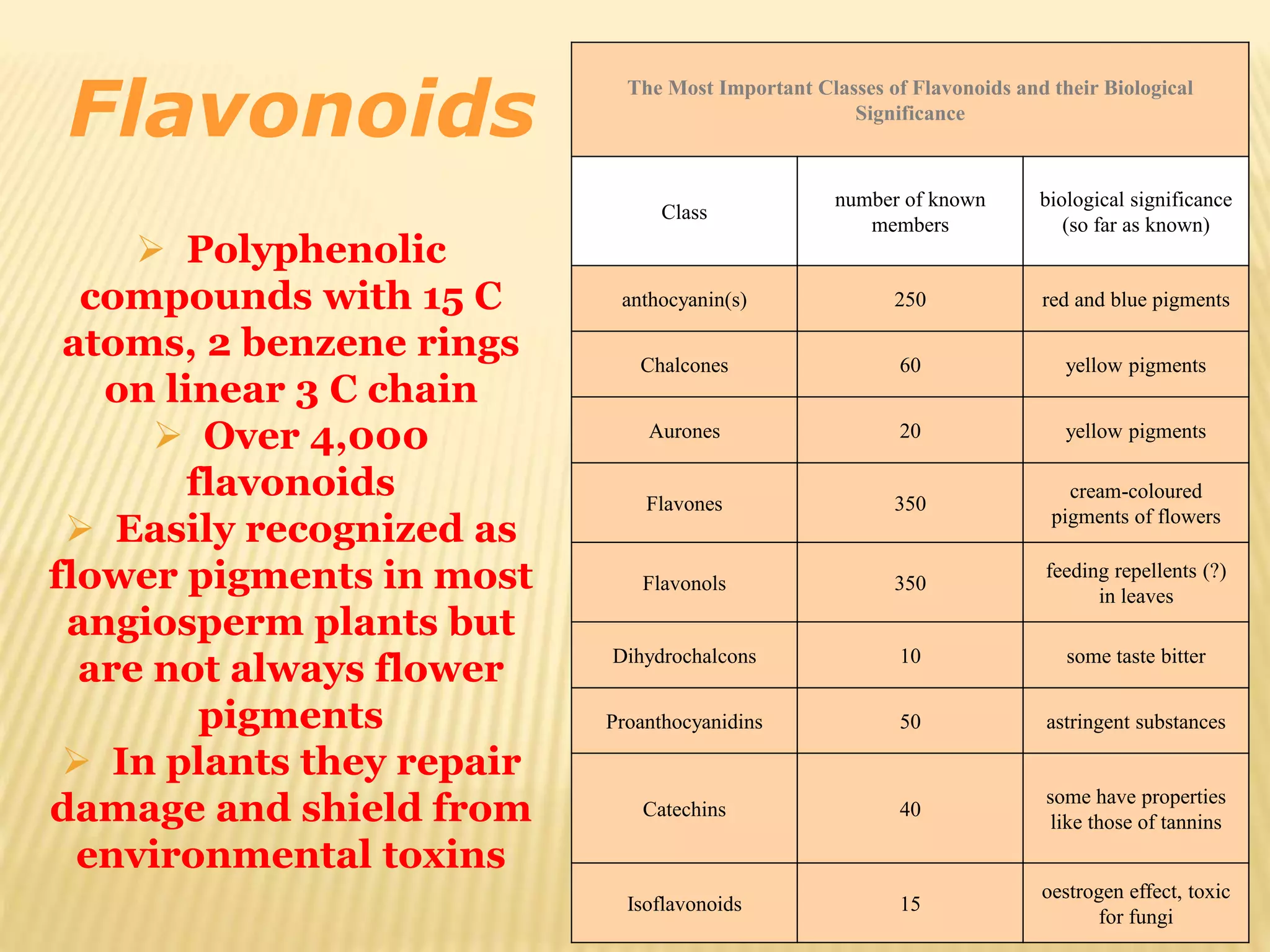
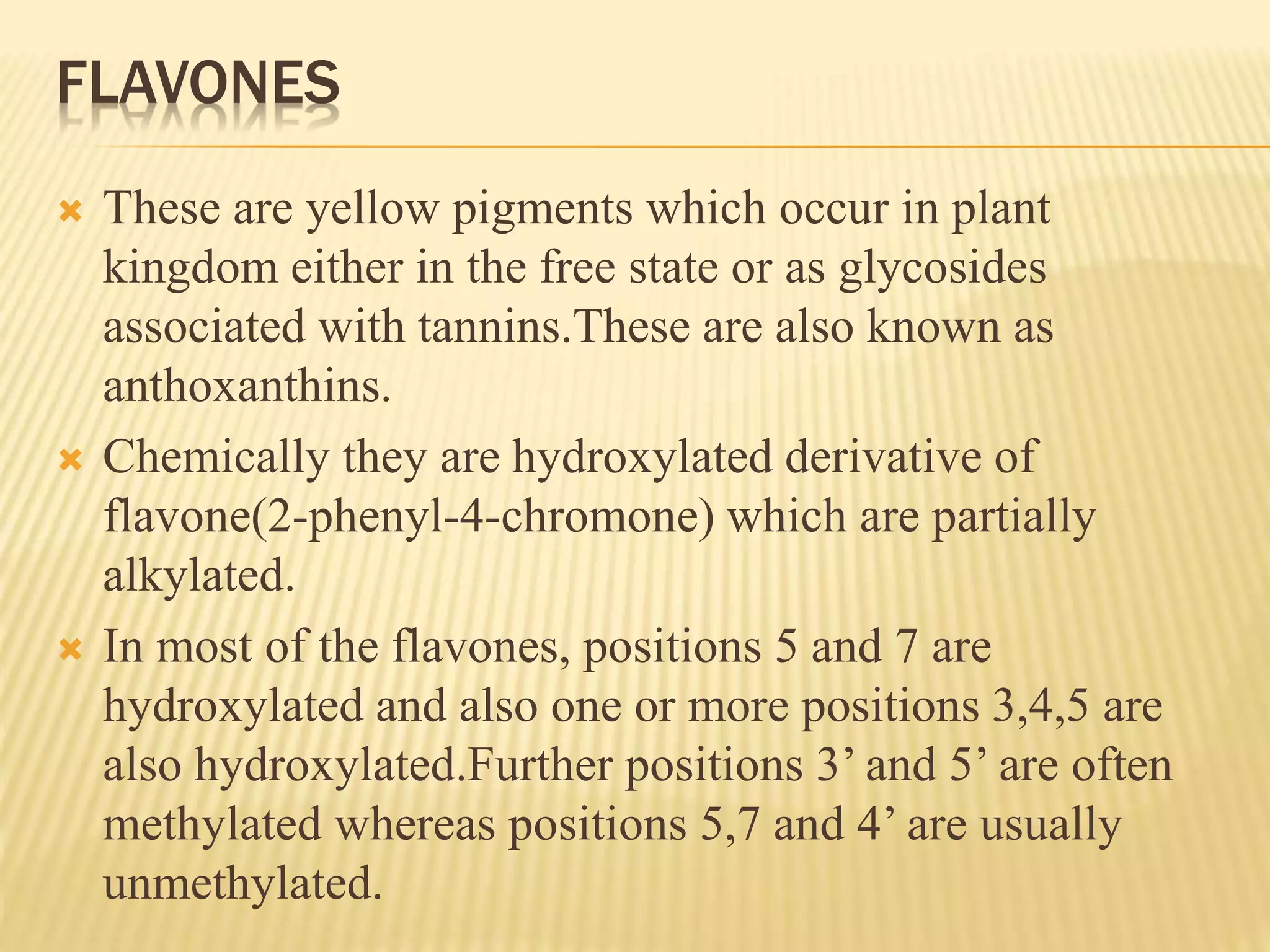
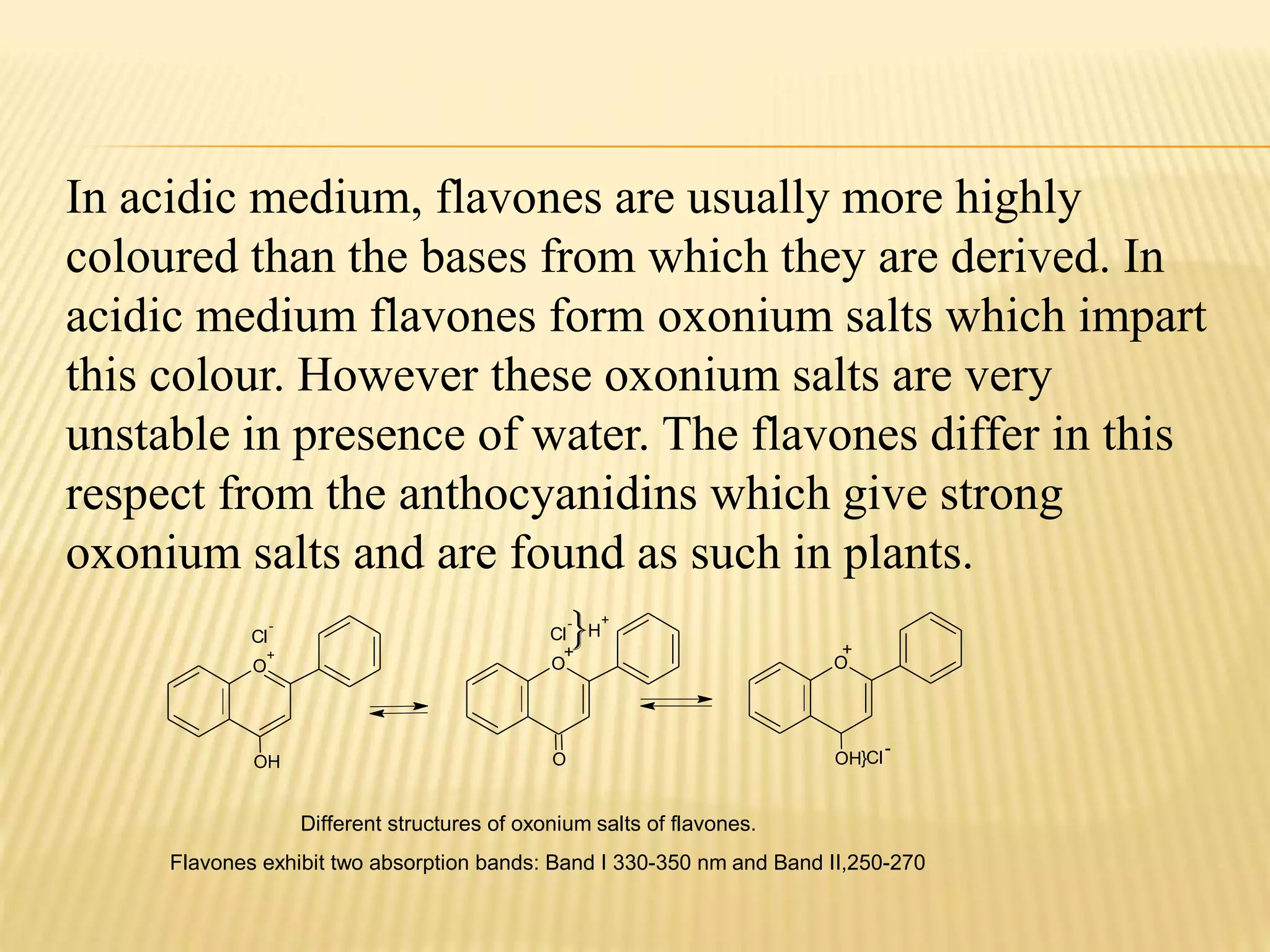
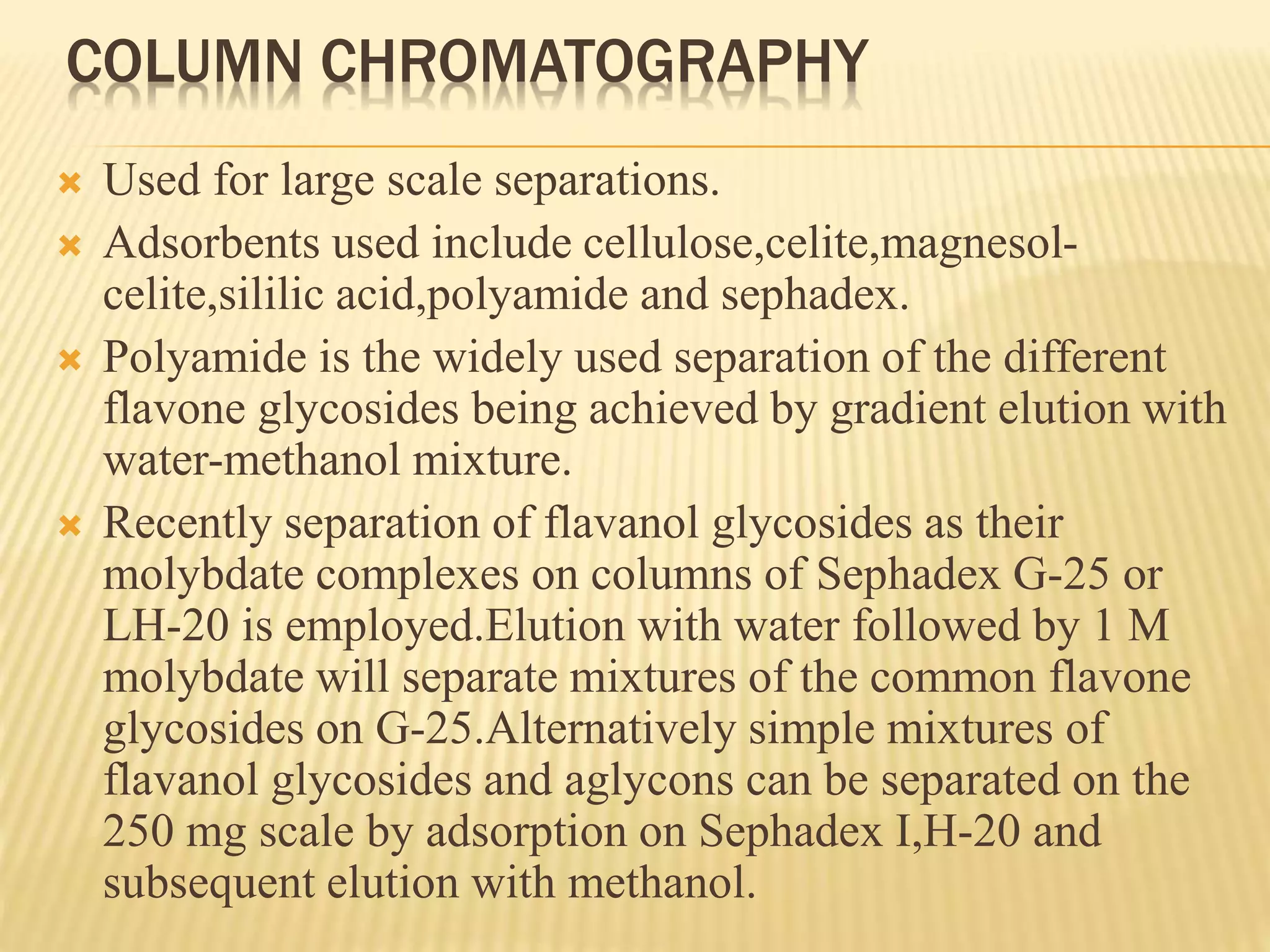
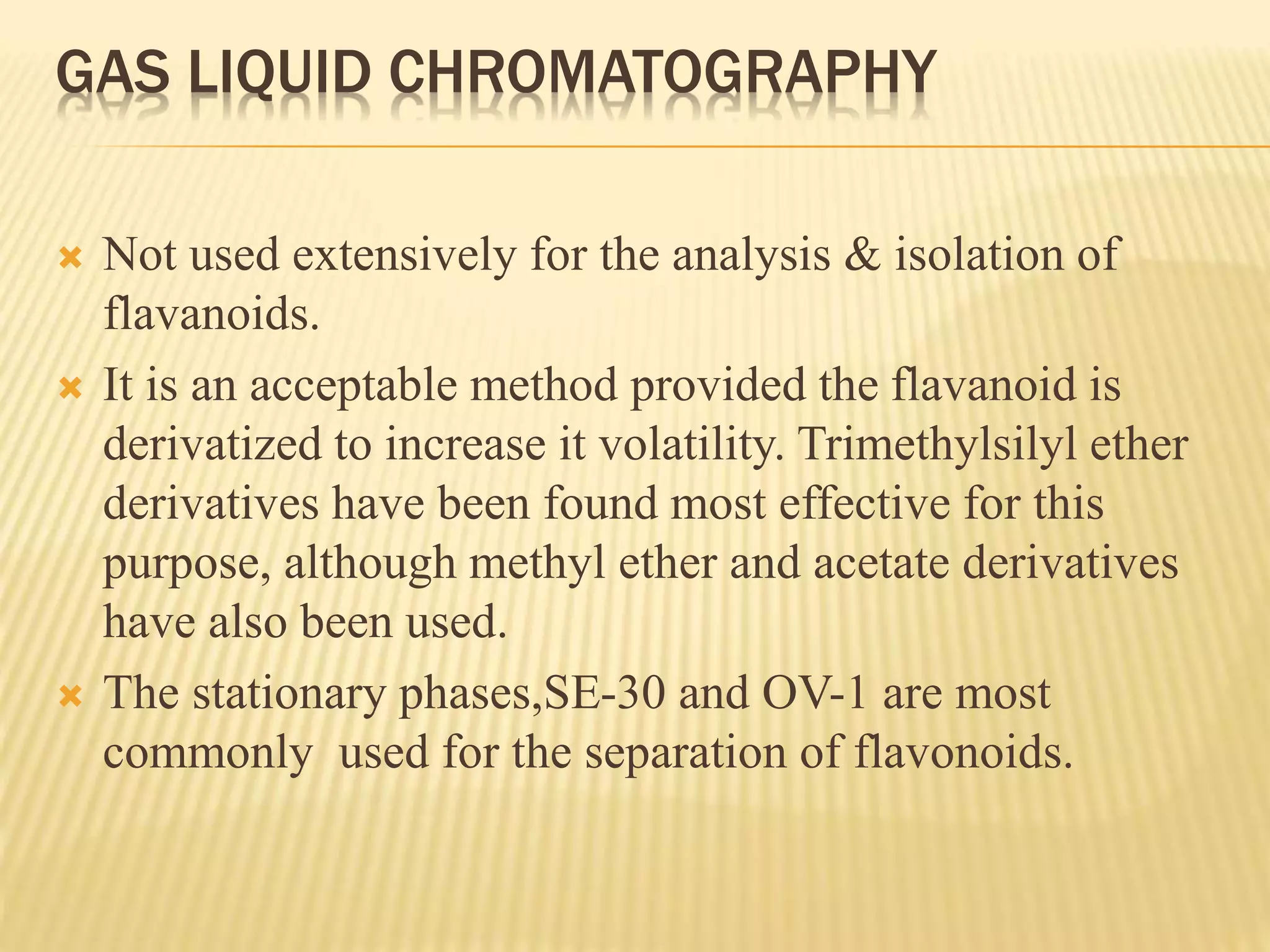
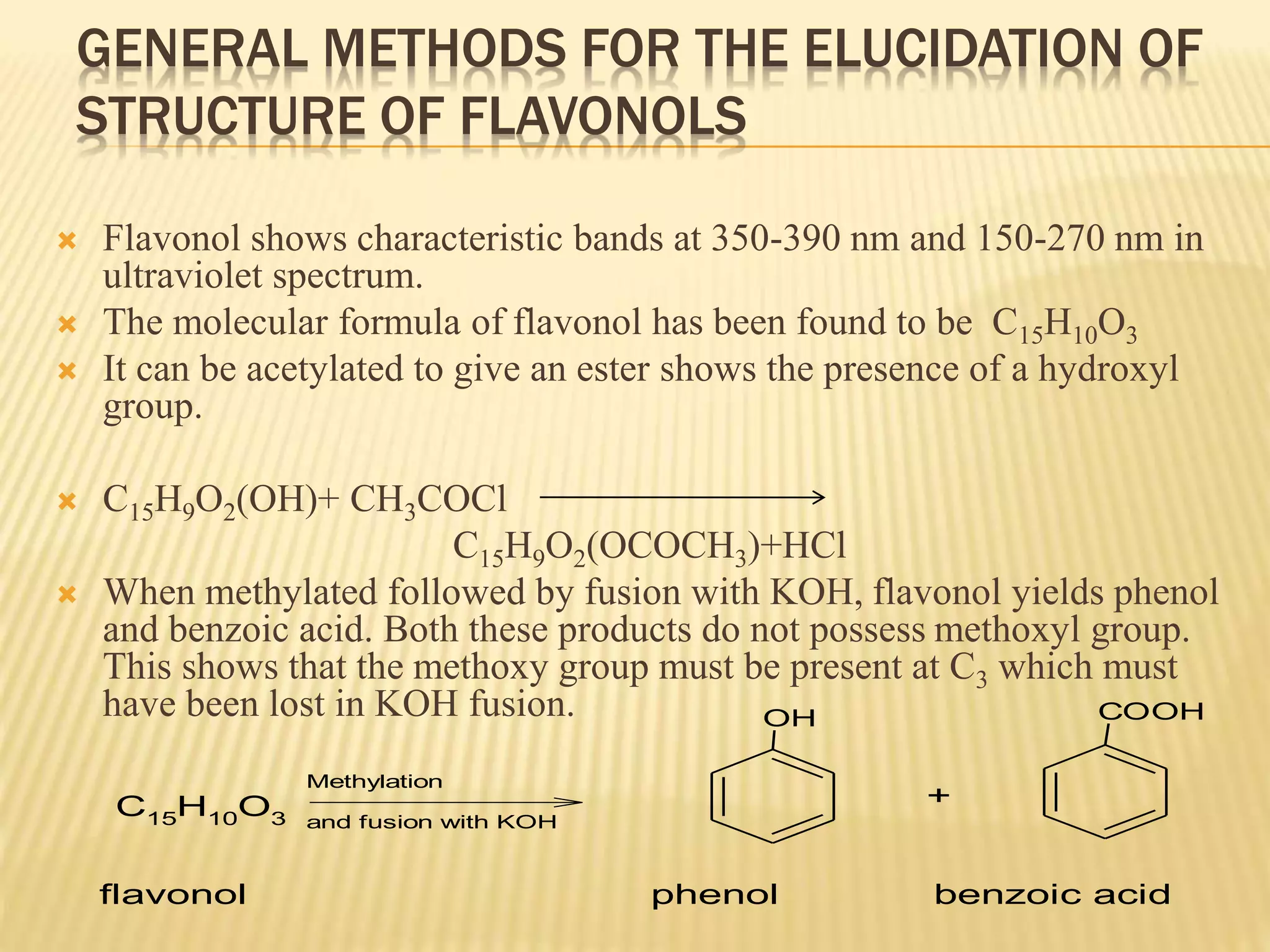
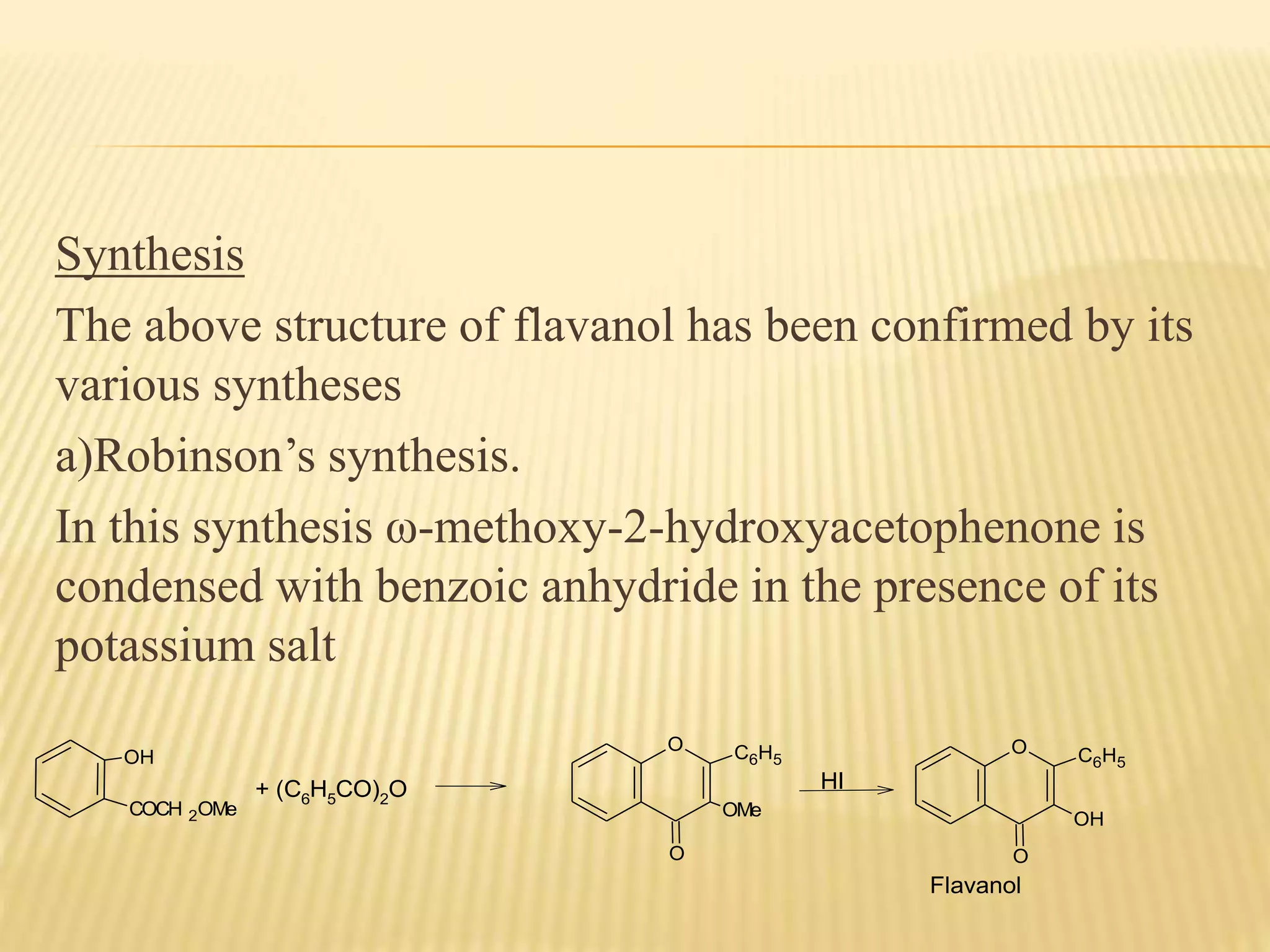
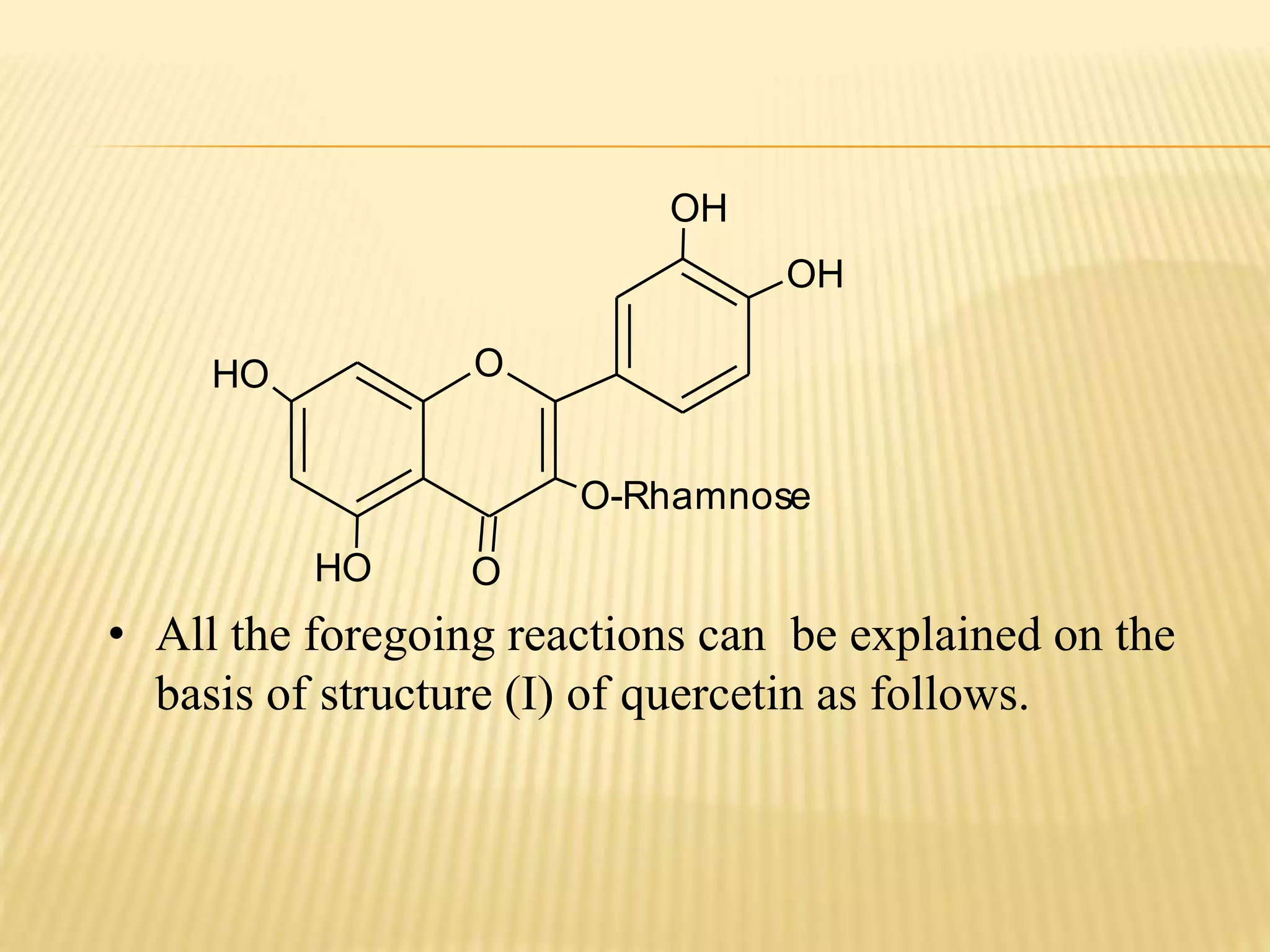
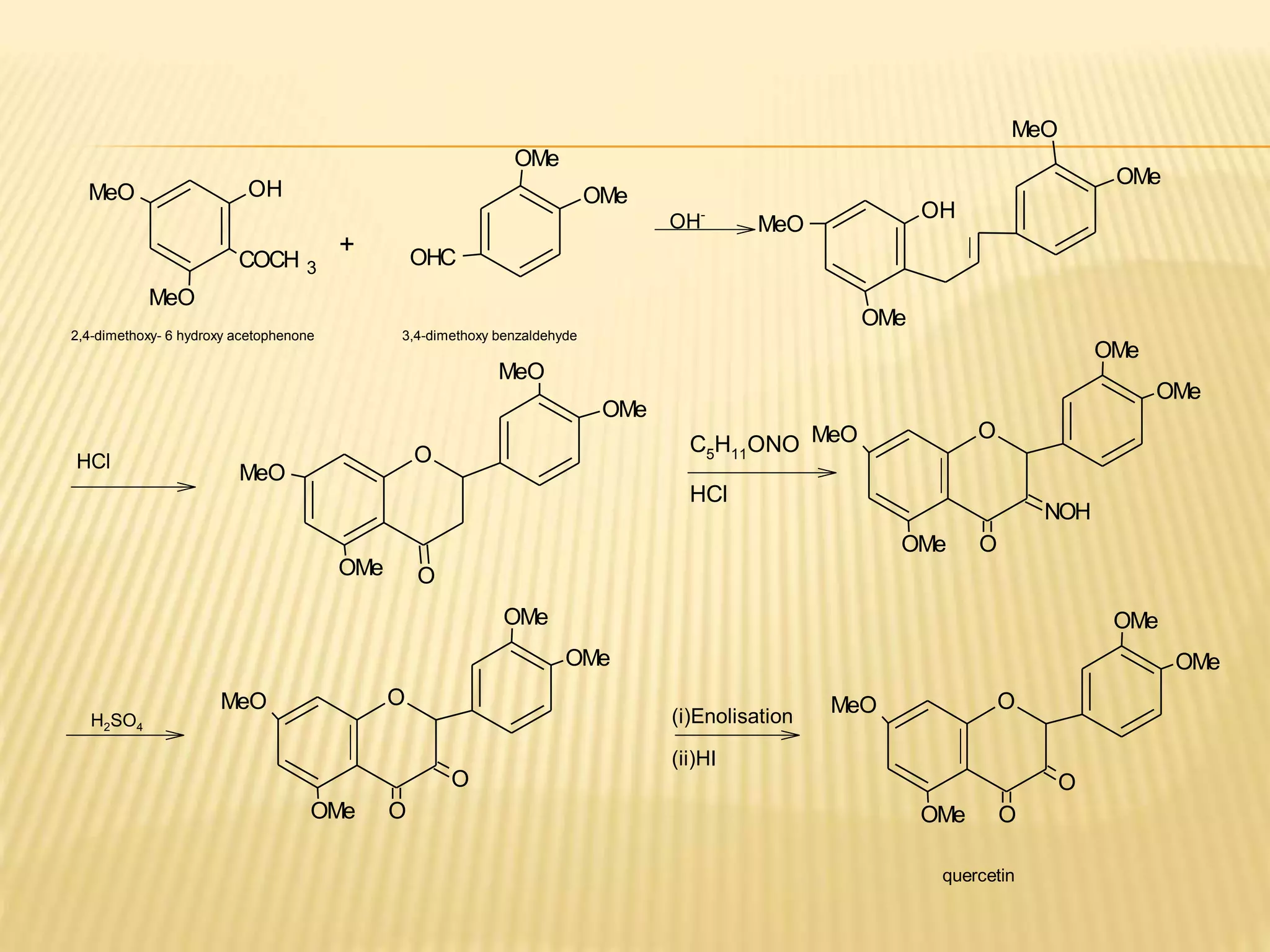
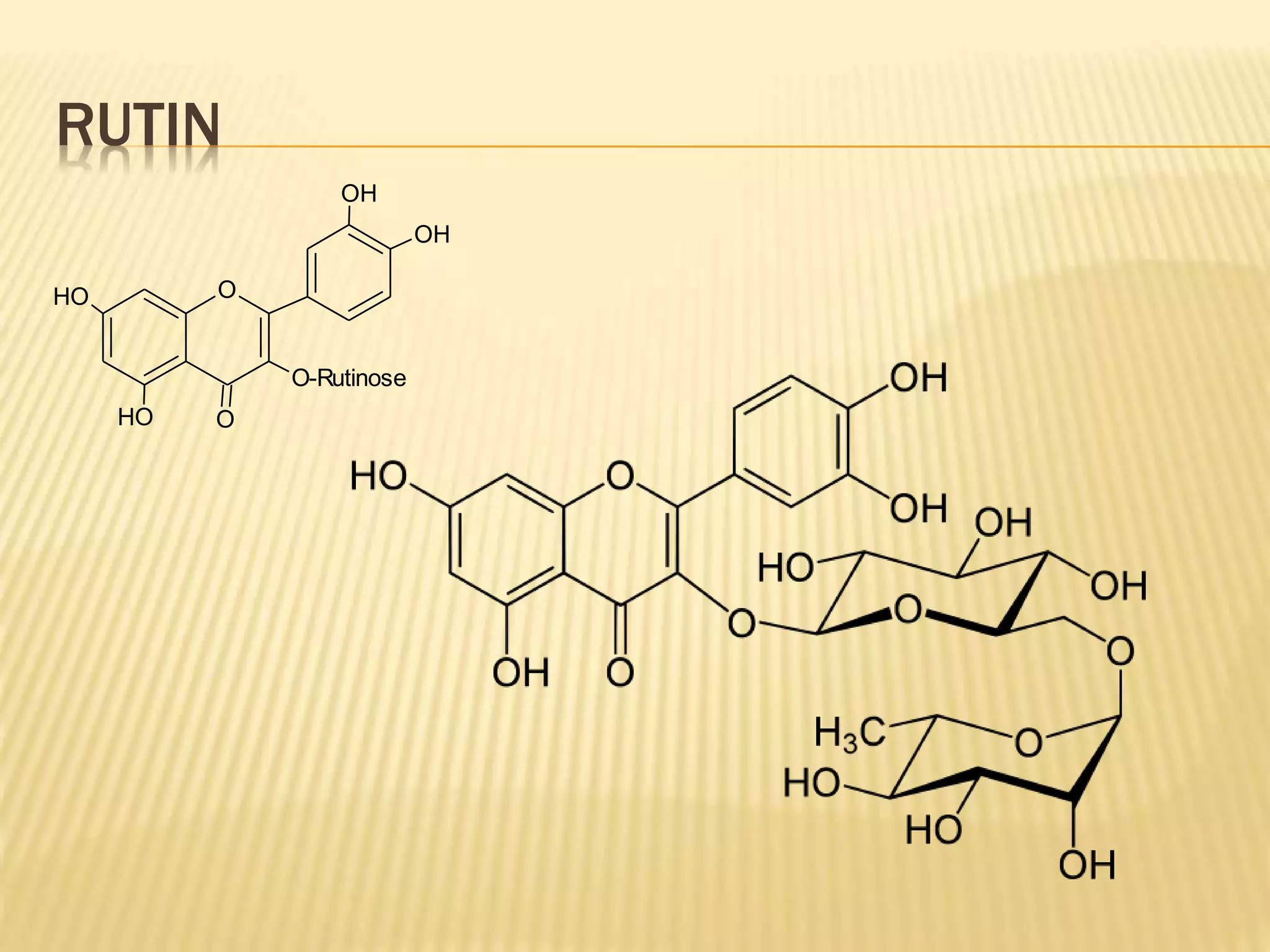
Flavonoids are polyphenolic compounds found in plants that act as antioxidants. They have 15 carbon atoms arranged in two benzene rings connected by a 3 carbon chain. There are over 4,000 known flavonoids that are commonly found as flower pigments but also occur in other plant parts. Major classes of flavonoids include flavones, flavonols, flavanones, and isoflavonoids. Flavonoids have important biological functions like repairing damage, protecting plants from toxins and UV radiation, and possess anti-inflammatory, antiviral and antitumor properties in humans.