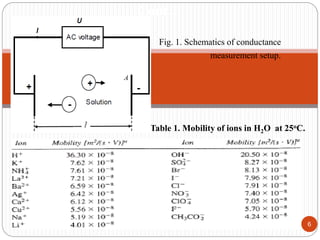
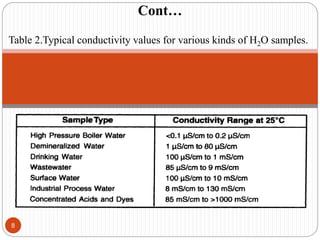
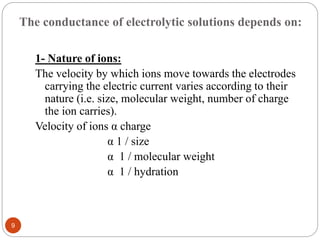
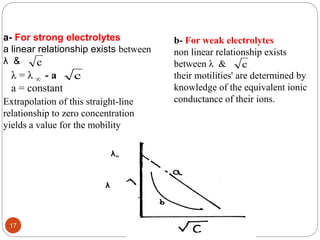
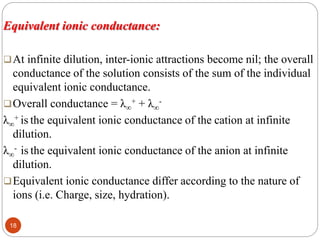
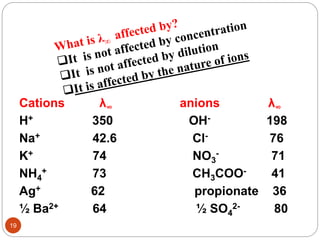
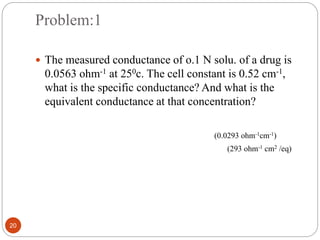
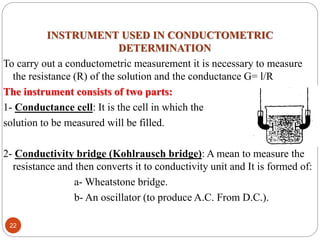
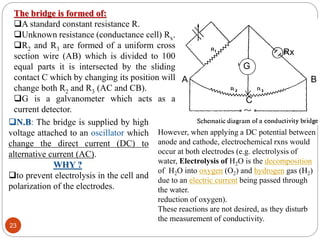
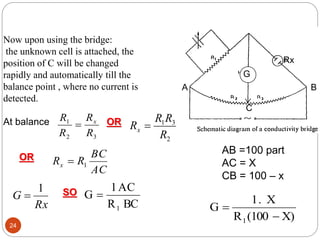
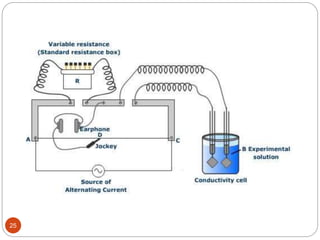
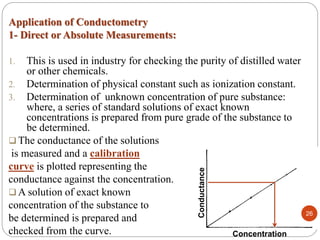
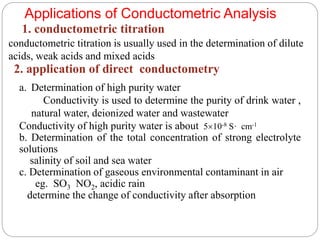
This document discusses conductometry, which is a method of analysis based on measuring the electrolytic conductance of a solution. It begins by classifying different electrochemical methods, including conductometry and electrophoresis which do not involve redox reactions. It then discusses key concepts in conductometry such as conductivity, conductance, equivalent conductance, and how various factors like ion nature, temperature, concentration, and electrode size affect conductance. It also provides examples of calculating conductance and equivalent conductance from experimental measurements. Instrumentation for conductometric determination includes a conductance cell and conductivity bridge.