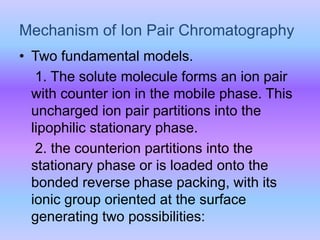
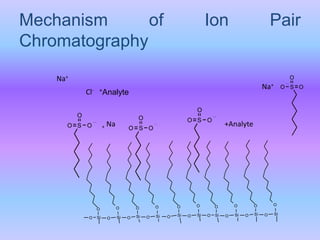
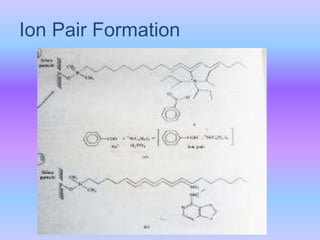
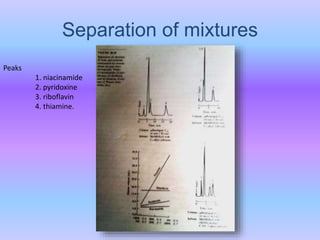
The document provides an overview of chromatography, particularly focusing on high performance liquid chromatography (HPLC) and ion pair chromatography. It explains the principles, mechanisms, and advantages of these techniques, including their applications in separating molecular mixtures, especially in pharmaceutical analysis. Various factors influencing retention behavior and separation efficiency are also discussed.




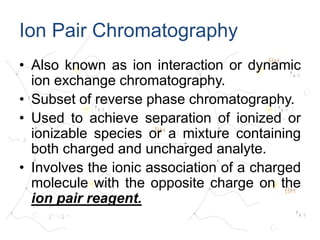
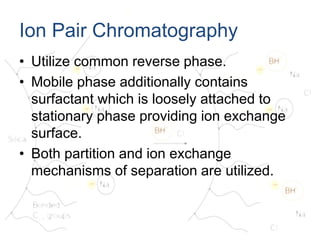
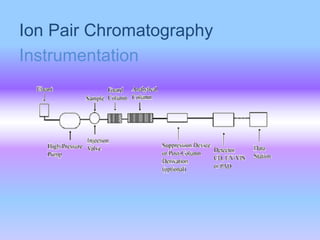
![• This charged stationary phase can then
retain and separate organic solute ions of
the opposite charge by forming a
reversible ion pair complex with the
ionized sample. eg
RCOO- + R4N+ --- [R4N+-OOCR] ion pair
Ion Pair Chromatography
Ion Pair Reagent](https://image.slidesharecdn.com/ionpairchromatography-abhishekrai-191115071951/85/Ion-pair-chromatography-for-pharmacy-students-10-320.jpg)