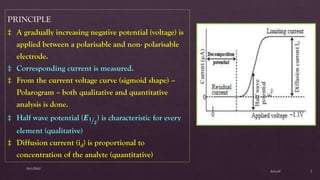
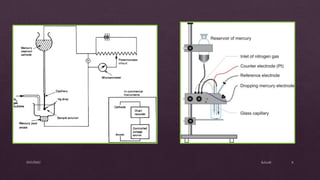
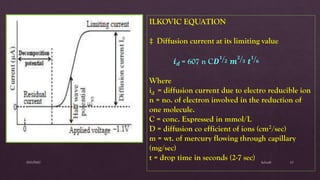
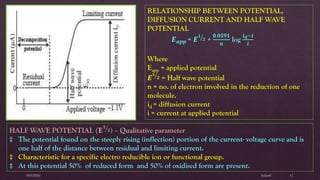
Polarography is an electroanalytical technique that involves measuring the current in a cell with a gradually increasing negative potential applied between a polarizable working electrode, usually a dropping mercury electrode, and a non-polarizable reference electrode. It can be used for both qualitative and quantitative analysis of electroreducible or electrooxidizable elements. The current-voltage curve obtained is called a polarogram, from which the half-wave potential and diffusion current can be determined for qualitative and quantitative analysis, respectively. Key components of the polarograph include the dropping mercury electrode, rotating platinum electrode, and reference electrode. Factors like concentration, temperature, viscosity, and capillary characteristics affect the diffusion current.